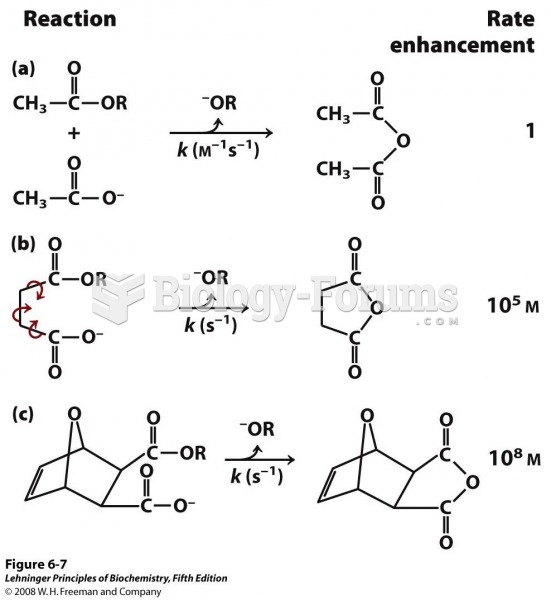
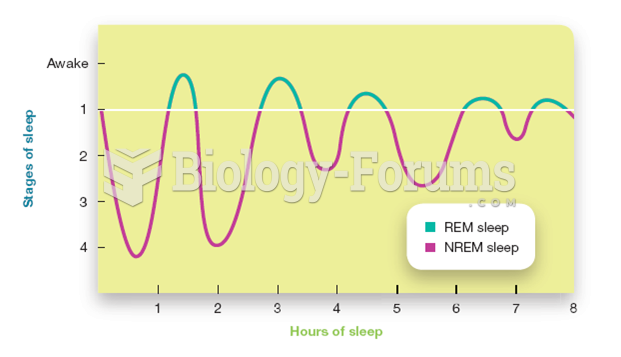
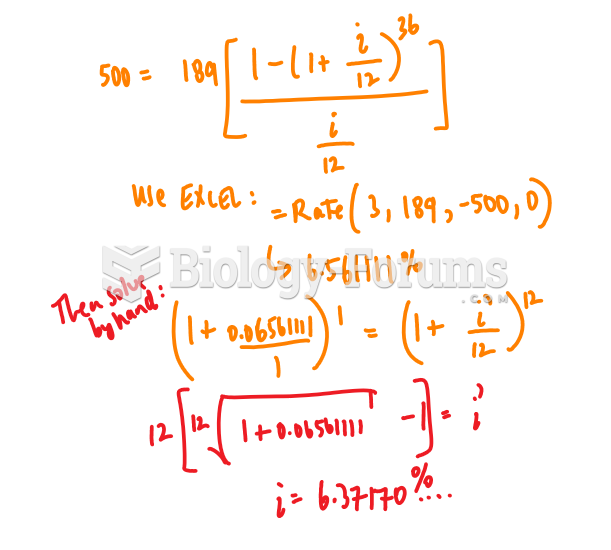
|
|
|
Children with strabismus (crossed eyes) can be treated. They are not able to outgrow this condition on their own, but with help, it can be more easily corrected at a younger age. It is important for infants to have eye examinations as early as possible in their development and then another at age 2 years.
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Medication errors are three times higher among children and infants than with adults.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.