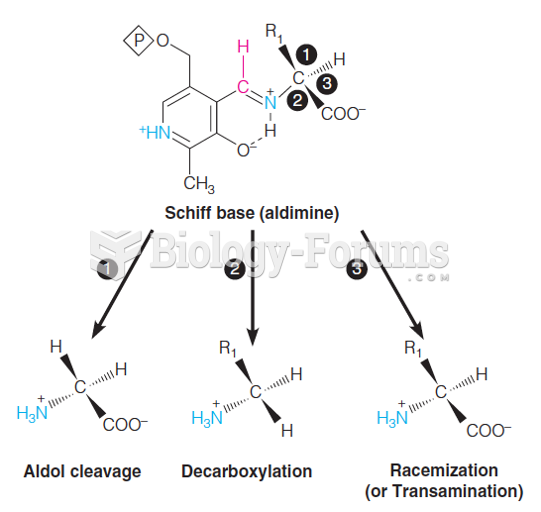
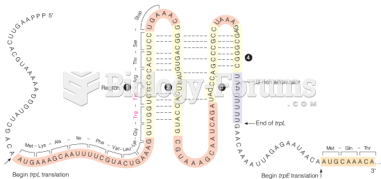
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
Did you know?
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.