This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
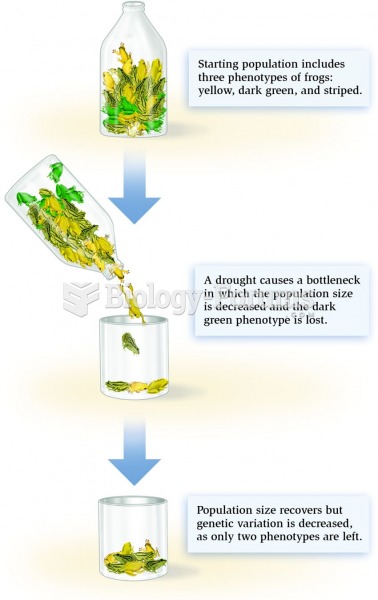
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Did you know?
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.
Did you know?
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).