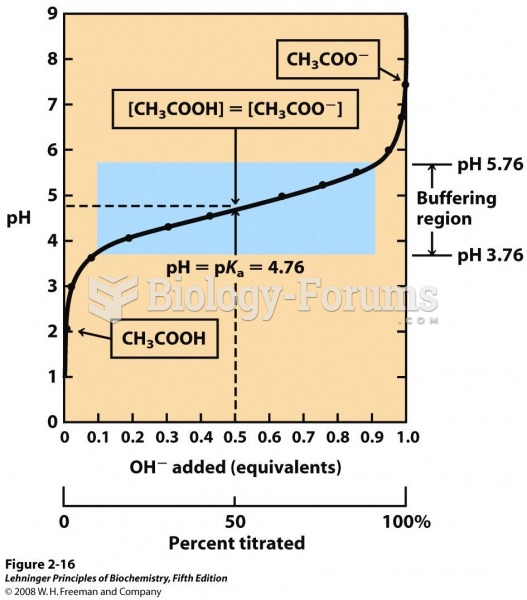
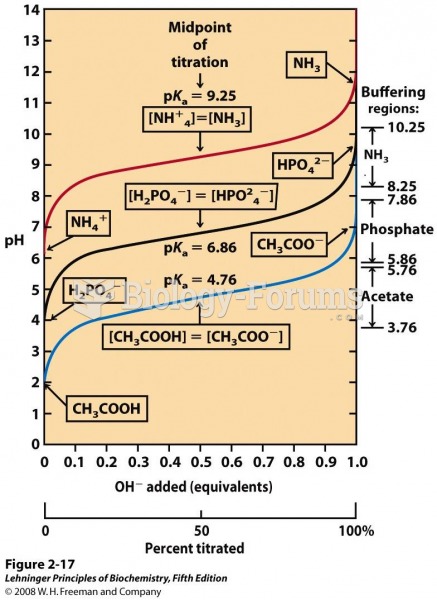
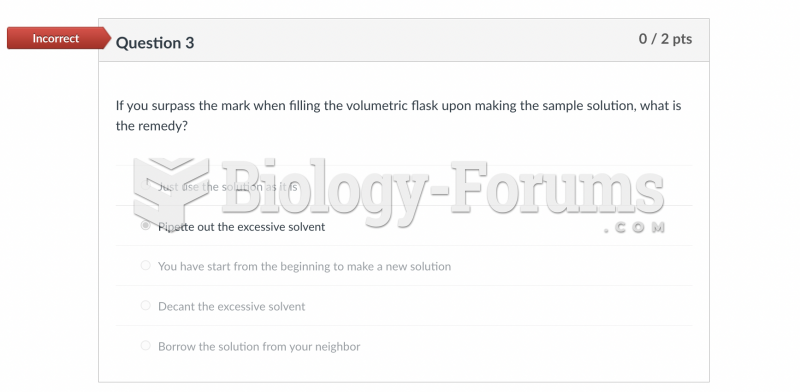
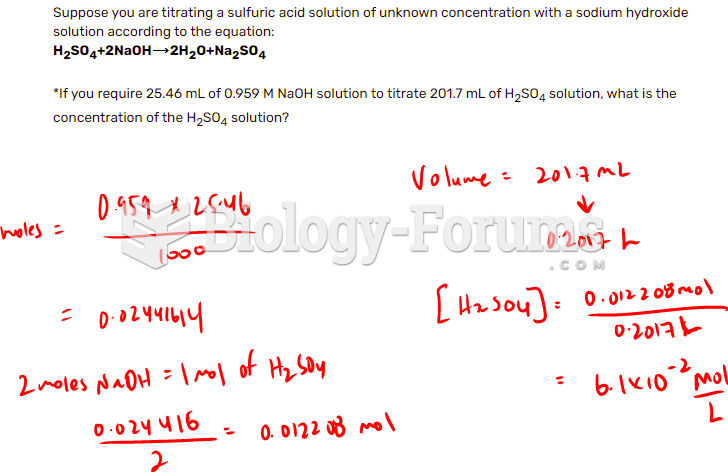
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.