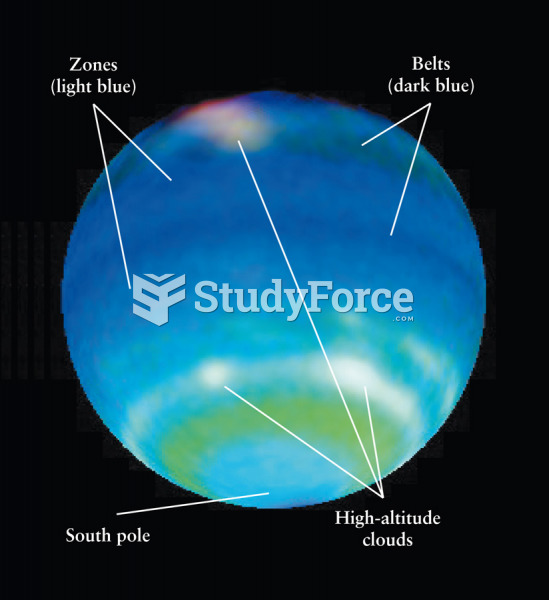
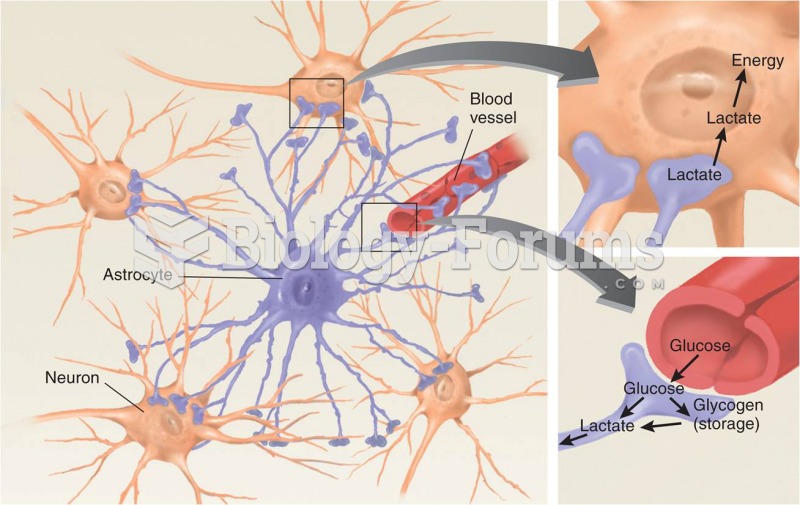
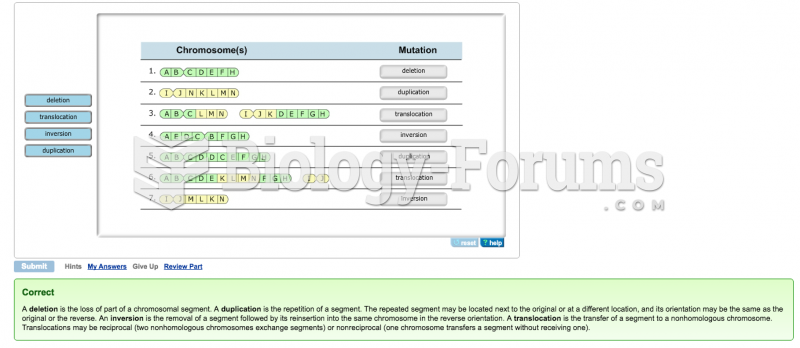
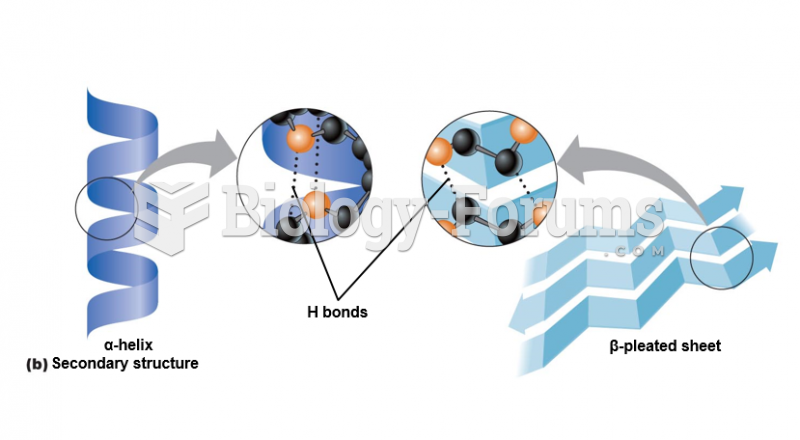
|
|
|
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
Vaccines prevent between 2.5 and 4 million deaths every year.
If you use artificial sweeteners, such as cyclamates, your eyes may be more sensitive to light. Other factors that will make your eyes more sensitive to light include use of antibiotics, oral contraceptives, hypertension medications, diuretics, and antidiabetic medications.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.