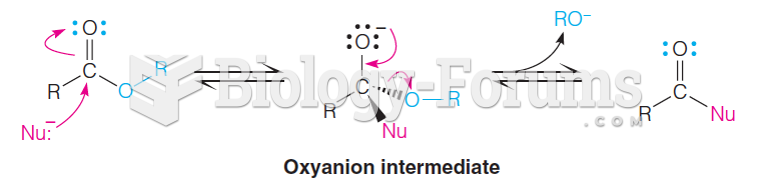
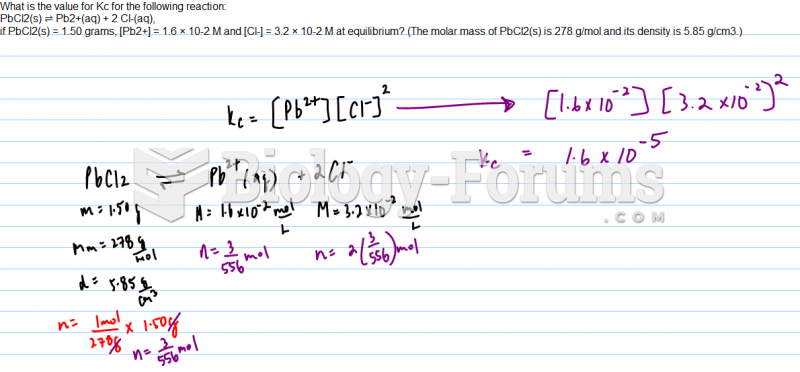
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Did you know?
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.