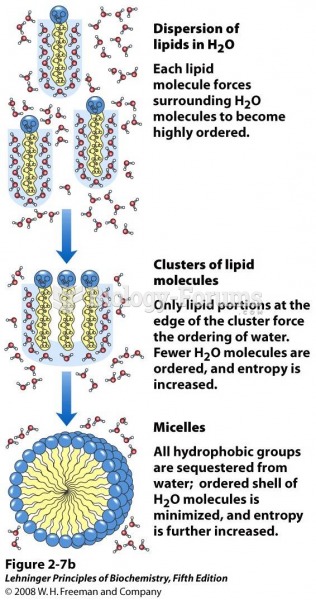
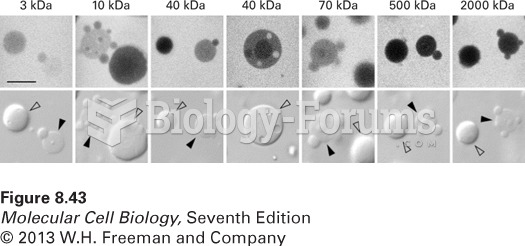
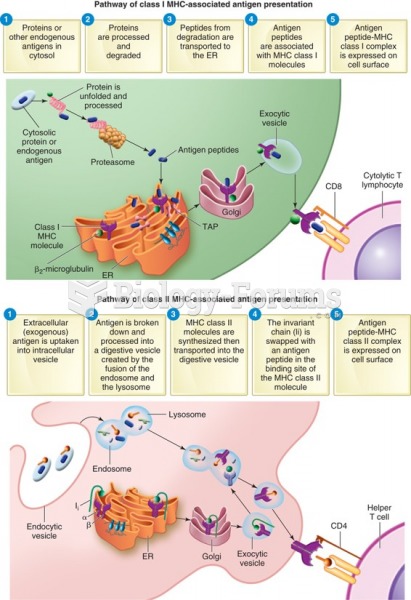
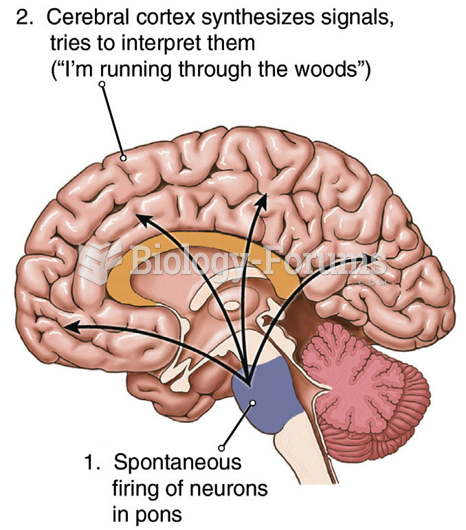
|
|
|
The largest baby ever born weighed more than 23 pounds but died just 11 hours after his birth in 1879. The largest surviving baby was born in October 2009 in Sumatra, Indonesia, and weighed an astounding 19.2 pounds at birth.
Excessive alcohol use costs the country approximately $235 billion every year.
According to the American College of Allergy, Asthma & Immunology, more than 50 million Americans have some kind of food allergy. Food allergies affect between 4 and 6% of children, and 4% of adults, according to the CDC. The most common food allergies include shellfish, peanuts, walnuts, fish, eggs, milk, and soy.
There are more sensory neurons in the tongue than in any other part of the body.
Medication errors are more common among seriously ill patients than with those with minor conditions.