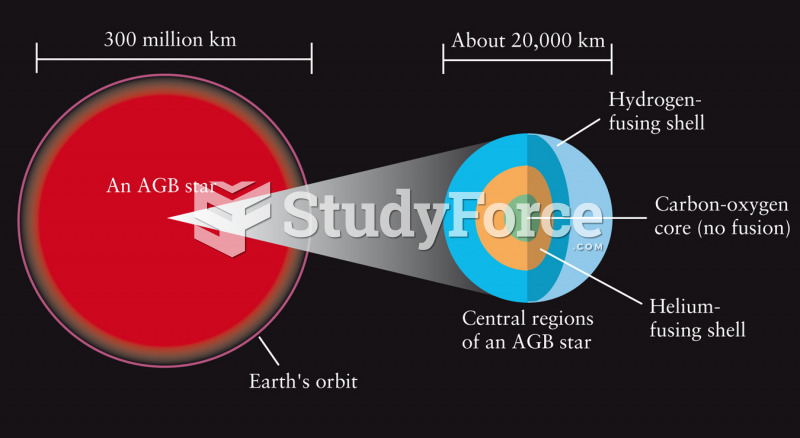
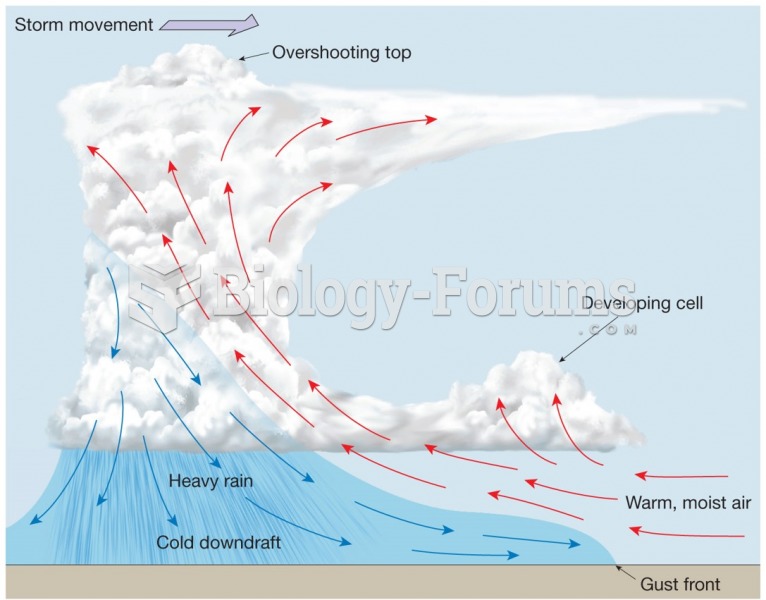
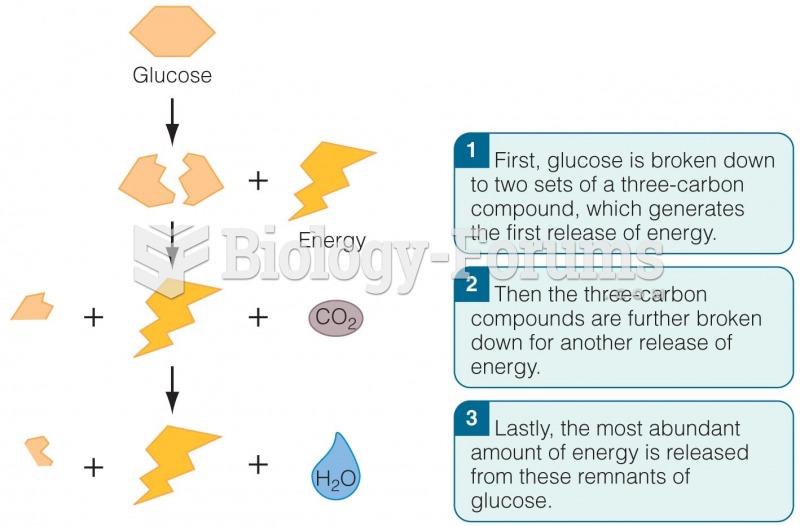
|
|
|
Did you know?
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Did you know?
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.