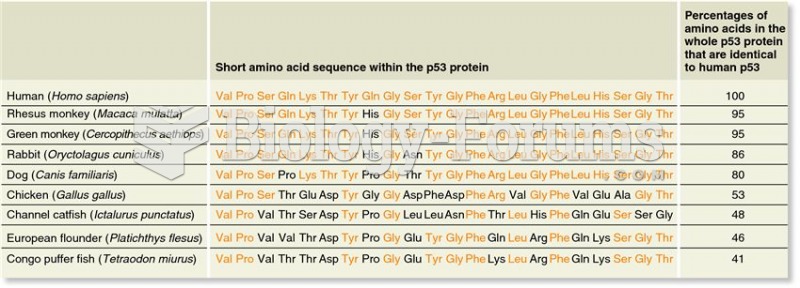
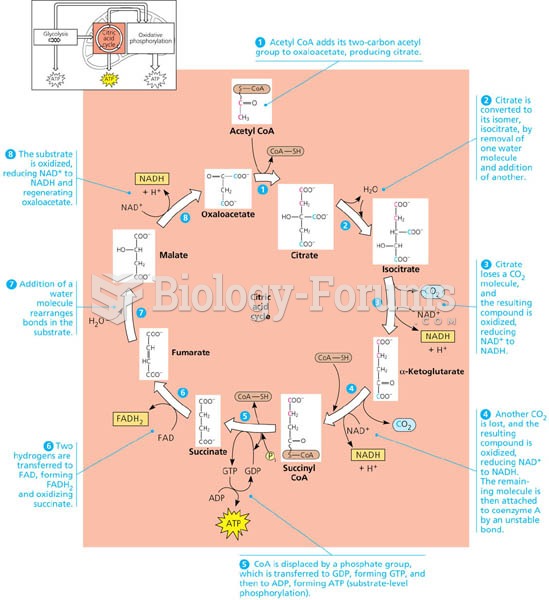
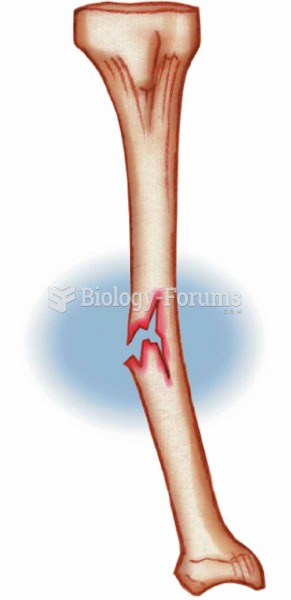
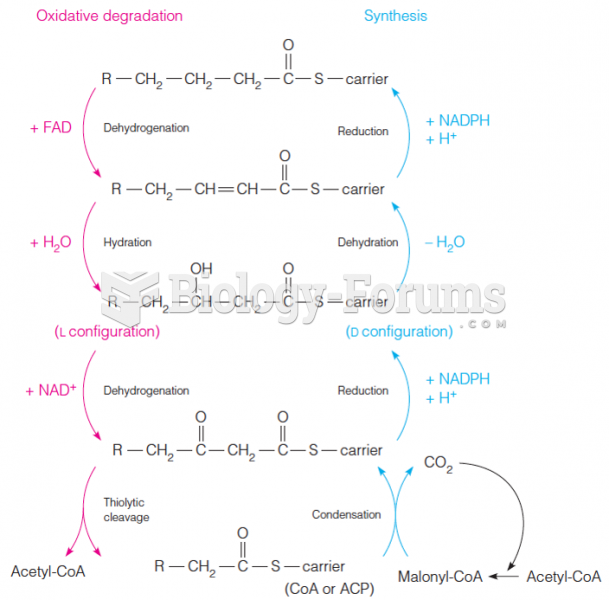
|
|
|
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Although the Roman numeral for the number 4 has always been taught to have been "IV," according to historians, the ancient Romans probably used "IIII" most of the time. This is partially backed up by the fact that early grandfather clocks displayed IIII for the number 4 instead of IV. Early clockmakers apparently thought that the IIII balanced out the VIII (used for the number 8) on the clock face and that it just looked better.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.