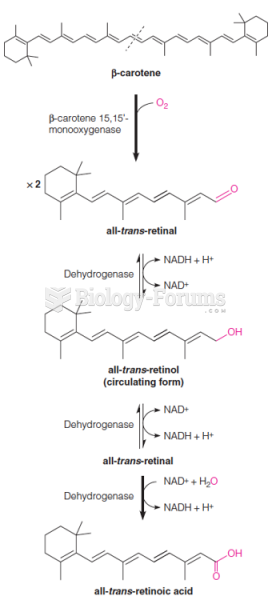
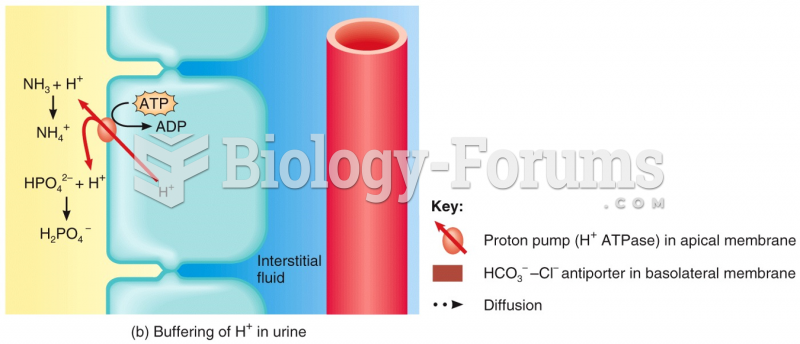
|
|
|
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Did you know?
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
Did you know?
Medication errors are three times higher among children and infants than with adults.