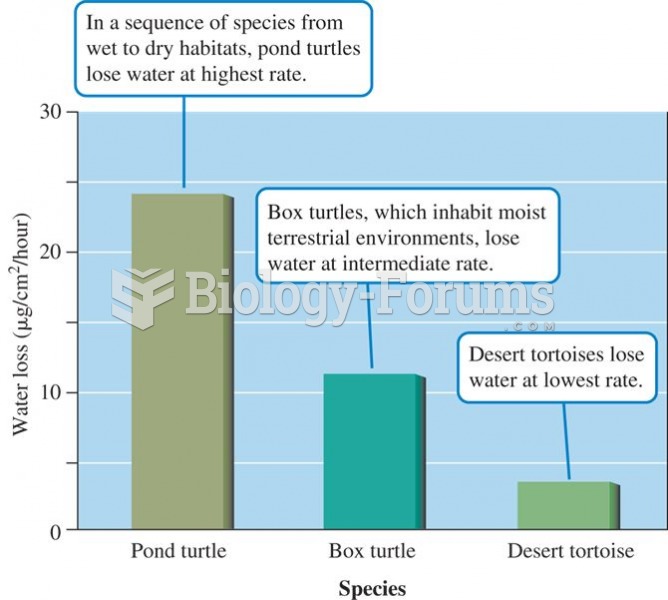
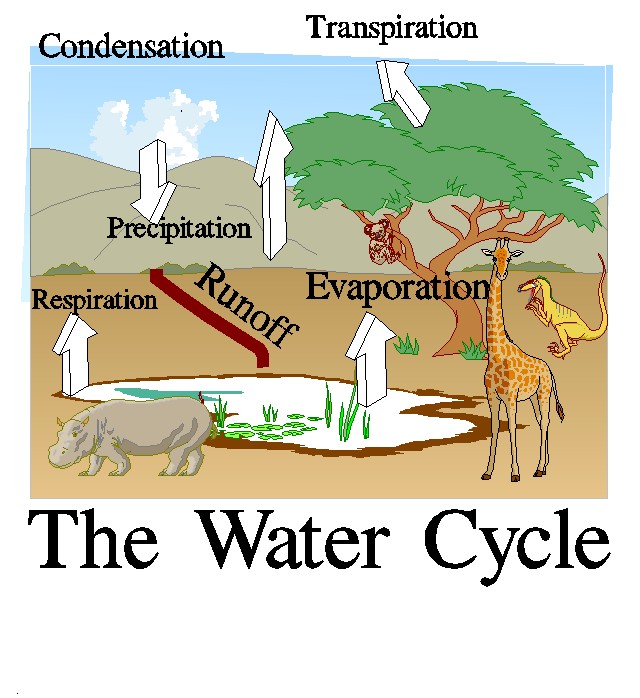
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.