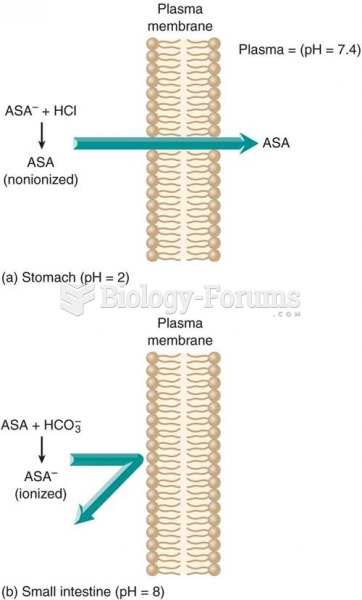
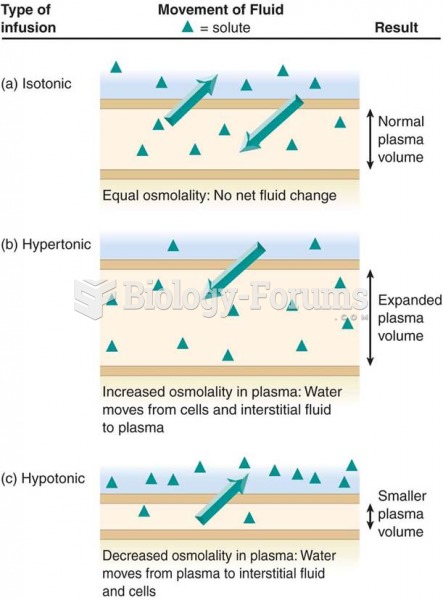
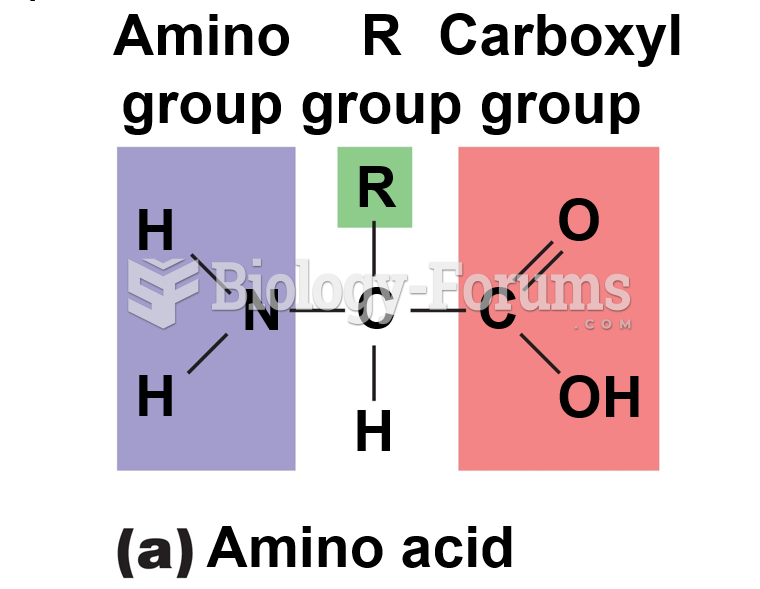
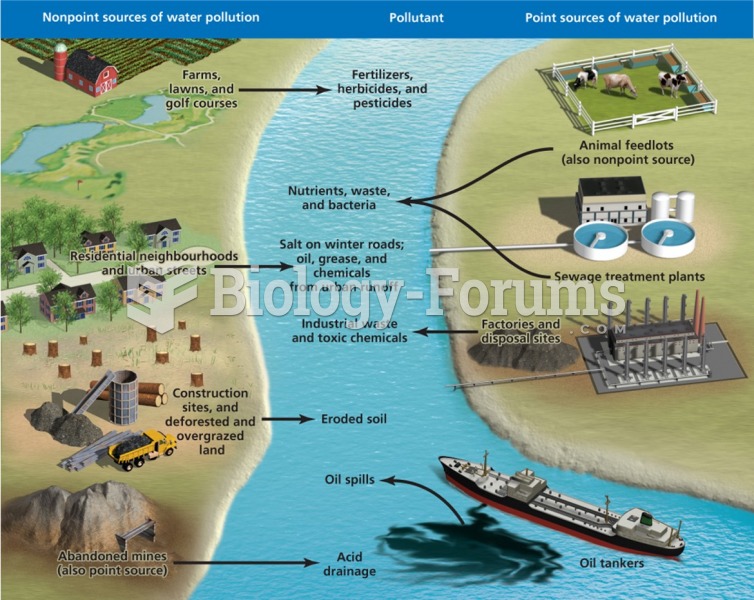
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
It is important to read food labels and choose foods with low cholesterol and saturated trans fat. You should limit saturated fat to no higher than 6% of daily calories.
Did you know?
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.
Did you know?
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.