|
|
|
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Blastomycosis is often misdiagnosed, resulting in tragic outcomes. It is caused by a fungus living in moist soil, in wooded areas of the United States and Canada. If inhaled, the fungus can cause mild breathing problems that may worsen and cause serious illness and even death.
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
 Sunlight on Mars is dimmer than on Earth. This photo of a Martian sunset was imaged by Mars Pathfind
Sunlight on Mars is dimmer than on Earth. This photo of a Martian sunset was imaged by Mars Pathfind
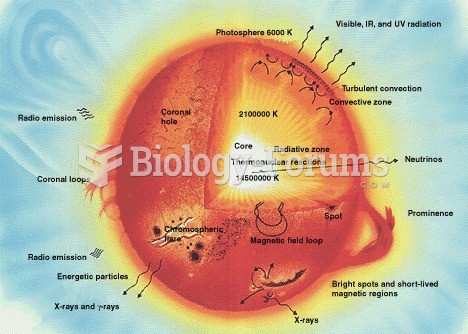 Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones
Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones