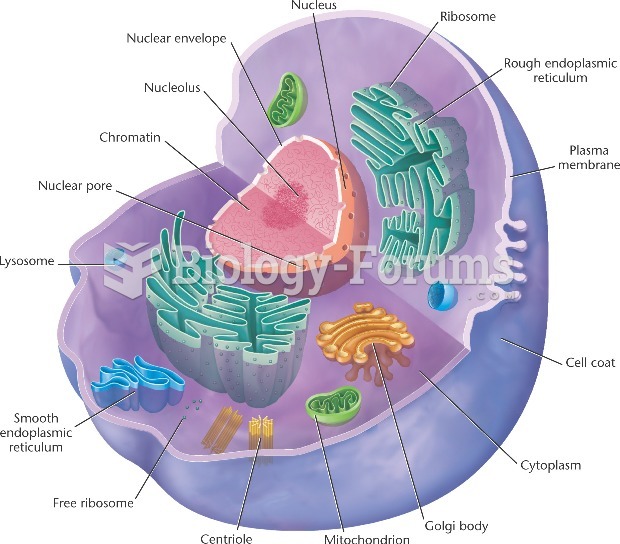
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Certain chemicals, after ingestion, can be converted by the body into cyanide. Most of these chemicals have been removed from the market, but some old nail polish remover, solvents, and plastics manufacturing solutions can contain these substances.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
Did you know?
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.