 |
| Previous Image | Next Image |
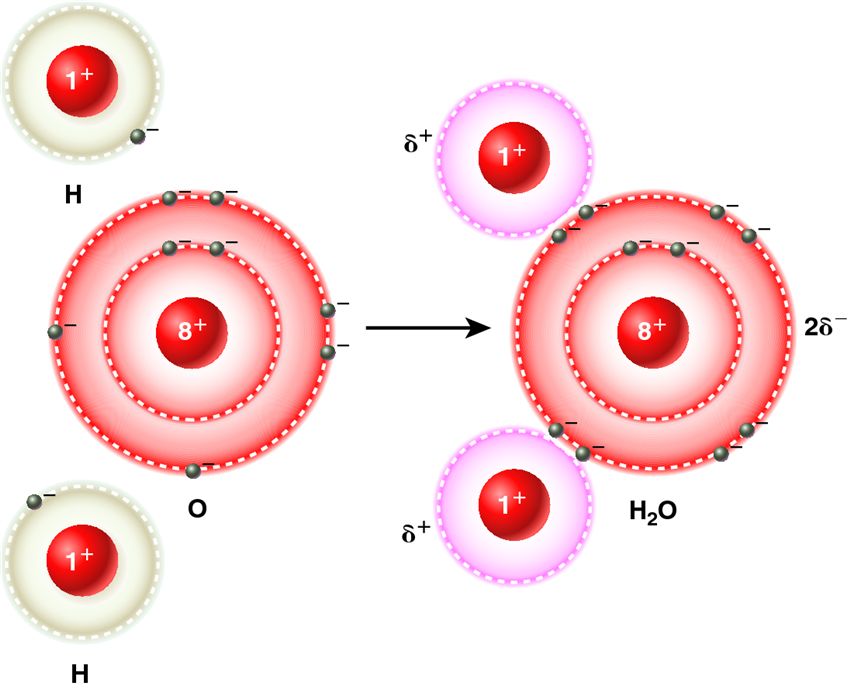
| Description: In a water molecule, two hydrogen atoms share electrons with an oxygen atom. Because oxygen has a higher electronegativity, the shared electrons spend more time closer to oxygen. This gives oxygen a partial negative charge, designated as 2[tex]{\delta}[/tex]?, and each hydrogen a partial positive charge, designated as [tex]{\delta}[/tex]+.
Picture Stats: Views: 4253 Filesize: 55.19kB Height: 689 Width: 850 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=142 Keywords: Polar covalent bonds in water molecules |
