 |
| Previous Image | Next Image |
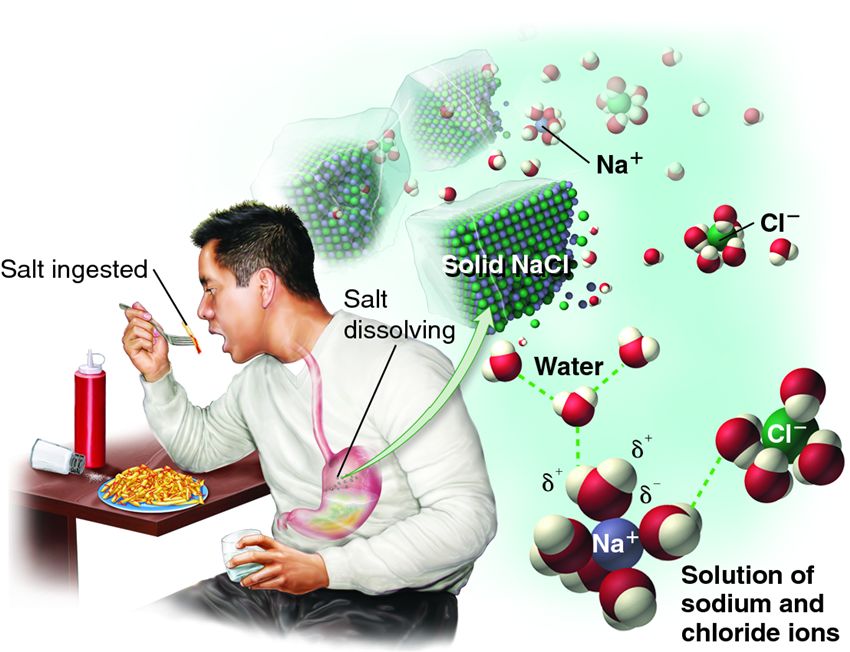
| Description: The ability of water to dissolve sodium chloride crystals depends on the electrical attraction between the polar water molecules and the charged sodium and chloride ions. Water molecules surround each ion as it becomes dissolved.
Picture Stats: Views: 1840 Filesize: 79.81kB Height: 652 Width: 850 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=150 Keywords: NaCl crystals dissolving in water |
