 |
| Previous Image | Next Image |
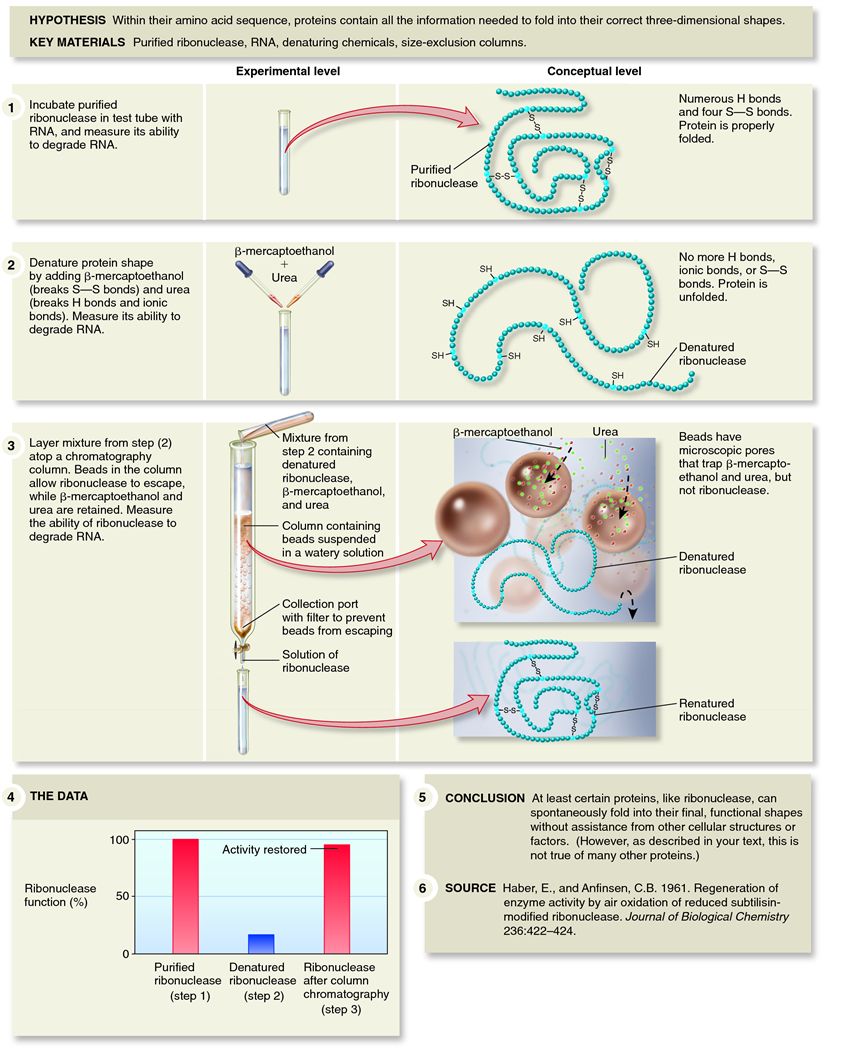
| Description: To test this hypothesis, Anfinsen studied ribonuclease at the National Institute of Health in the United States. Ribonuclease is an abundant enzyme that degrades RNA. Biochemists had already determined that ribonuclease has four disulphide bonds between eight cysteine amino acids. Anfinsen began with purified ribonucleaseâ€â€Âthis approach is called an in vitro experiment, meaning “done in glass,†as in a test tube. The key point is that other cellular components were not present, only the purified protein. He exposed ribonuclease to a chemical called urea that disrupted the hydrogen and ionic bonds, and to another chemical called ?-mercaptoethanol that broke the S S bonds. Following this treatment, he measured the ability of the treated enzyme to degrade RNA. The enzyme had lost nearly all of its ability to degrade RNA. The key step in this experiment came when Anfinsen removed the urea and ?-mercaptoethanol from the solution. Because these molecules are much smaller than ribonuclease, removing them from the solution was accomplished with a technique called dialysis. In dialysis, solutions are placed in a synthetic bag with microscopic pores that permit small molecules to pass through and leave the bag but retain large macromolecules, such as ribonuclease. Anfinsen placed the entire bag in a large beaker of water, into which the urea and ?-mercaptoethanol diffused. Then he retested the ribonuclease. The result revolutionized our understanding of proteins. The activity of the ribonuclease was almost completely restored! This meant that even in the complete absence of any cellular factors or organelles, an unfolded protein can refold into its functional structure. This was later confirmed by chemical analyses that demonstrated that the disulphide bonds had re-formed at the proper locations. Since Anfinsen's time, we have also learned that his experiments with ribonuclease are not representative of all proteins. Some proteins do require certain enzymes and other proteins to assist them in their proper folding. Nonetheless, Anfinsen's experiments provided compelling evidence that the primary structure of a polypeptide is the key determinant of a protein's tertiary structure, a correct observation that earned him a Nobel Prize in 1972. Picture Stats: Views: 4628 Filesize: 147.21kB Height: 1049 Width: 850 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=173 Keywords: Anfinsen's experiments with ribonuclease demonstrating that the primary structure of a polypept |
