 |
| Previous Image | Next Image |
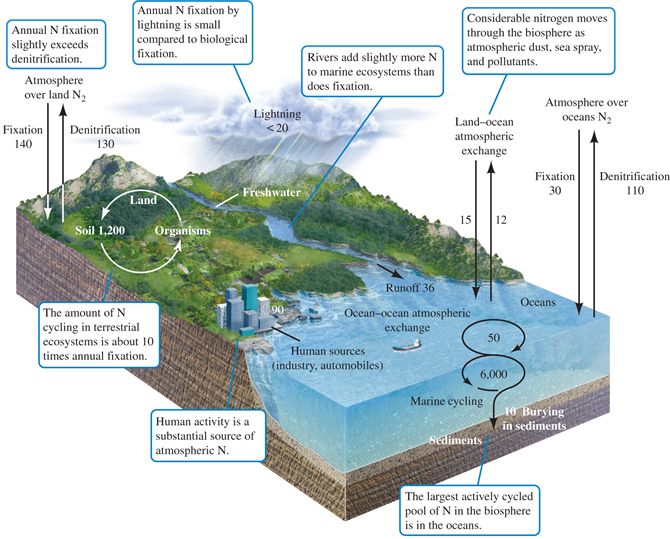
| Description: Nitrogen is important to the structure and functioning of organisms, forming part of key biomolecules such as amino acids, nucleic acids, and the porphyrin rings of chlorophyll and hemoglobin. In addition, as we saw in chapter 19, nitrogen supplies may limit rates of primary production in marine and terrestrial environments. Much of modern agriculture attempts to overcome nitrogen limitation through elaborate fertilization programs. Nitrogen is also released into the atmosphere through the burning of coal (along with sulfur), contributing to acidification of many lakes in Canada and the United States. Because of its importance and relative scarcity, nitrogen has drawn a great deal of attention from ecosystem ecologists. Page 509 Two critical aspects of the nitrogen cycle are mineralization and immobilization. Mineralization is the conversion of organic forms of nitrogen (e.g., proteins) into mineral forms (e.g., ammonia and nitrate). Immobilization is the reverse process, the conversion of mineral forms of nitrogen into organic forms. Because mineral nitrogen is often limiting plant growth, the balance between these processes can greatly alter productivity. Microbes play a central role in nitrogen cycling. The nitrogen cycle includes a major atmospheric pool in the form of molecular nitrogen, N2 (fig. 20.3). However, only a few organisms, all prokaryotes, can use this atmospheric supply of molecular nitrogen directly. These organisms, called nitrogen fixers, include (1) some cyanobacteria, or bluegreen algae, of freshwater, marine, and soil environments, (2) free-living soil bacteria such as Azotobacter and Azospirillum, (3) Rhizobia bacteria associated with the roots of leguminous plants, and (4) Frankia bacteria, associated with the roots of alders and several other species of woody plants (fig 20.4). It is important to recognize that plants themselves can not fix nitrogen, and instead rely on the activity of other species to provide mineralized nitrogen.
Picture Stats: Views: 2540 Filesize: 77.51kB Height: 539 Width: 670 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=2039 |
