 |
| Previous Image | Next Image |
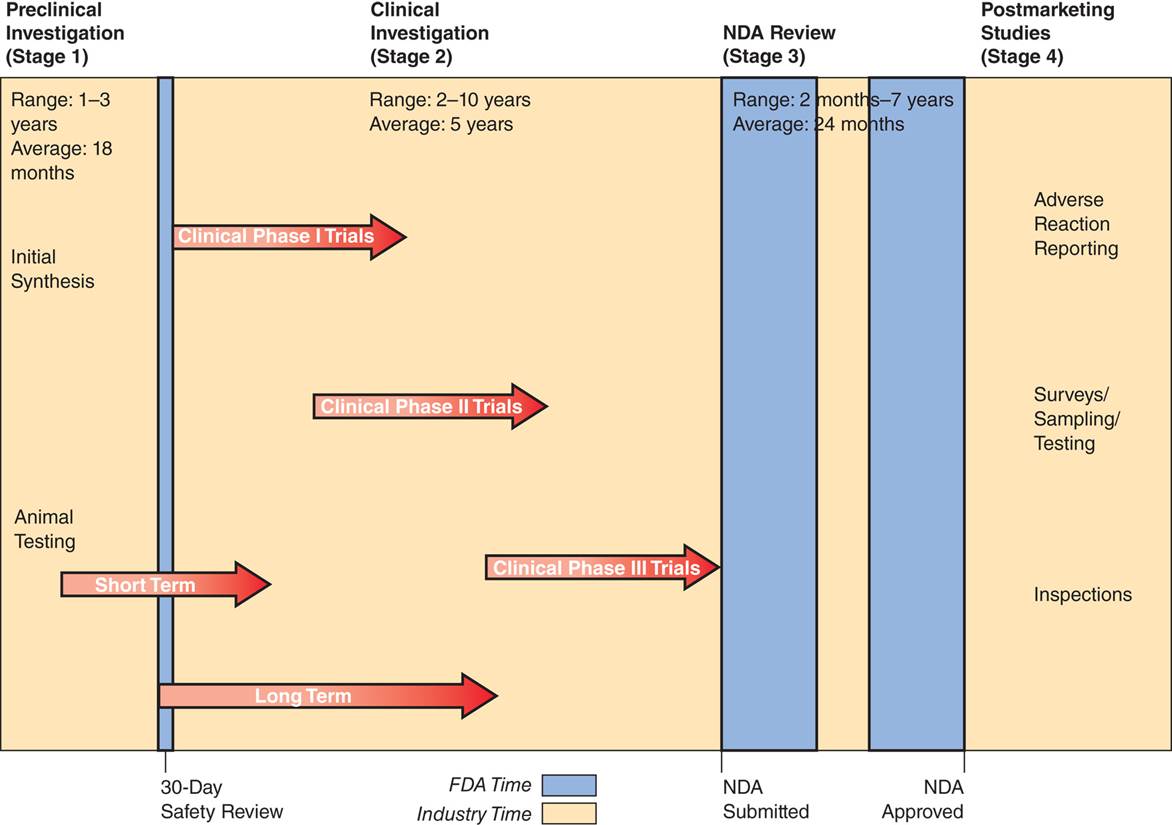
| Description: The approval of a new drug is a four-stage process: (1) preclinical investigation, (2) clinical investigation, (3) NDA submission and review, and (4) postmarketing studies. Within the second stage (clinical investigation), three phases of trials are conducted over two to ten years. Postmarketing studies, also called postmarketing surveillance, continue in large patient groups during the fourth stage of drug development.
Picture Stats: Views: 1419 Filesize: 72.66kB Height: 825 Width: 1172 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=21220 |
