 |
| Previous Image | Next Image |
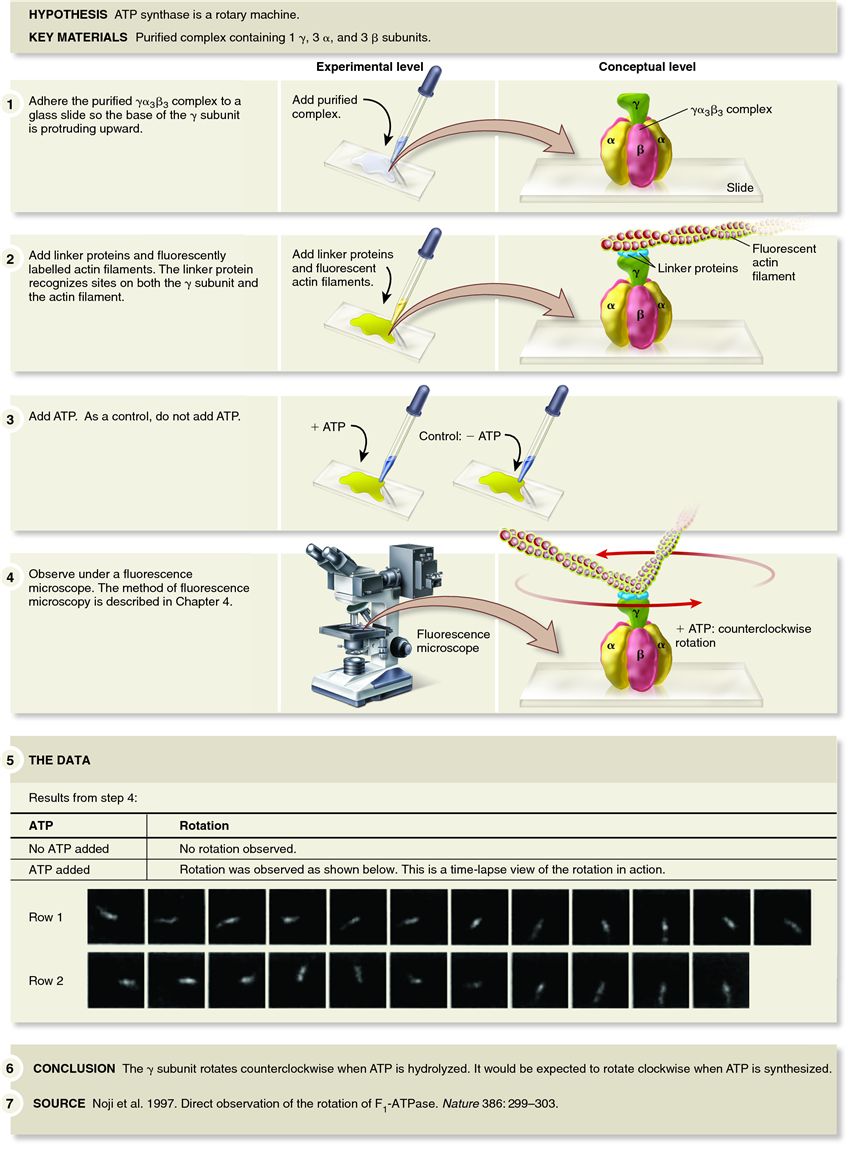
| Description: In 1997, Masasuke Yoshida, Kazuhiko Kinosita, and their colleagues set out to experimentally visualize the rotary nature of the ATP synthase. The membrane-embedded region of the ATP synthase can be separated from the rest of the protein by treatment of mitochondrial membranes with a high concentration of salt, releasing the portion of the protein containing the one ?, three ?, and three ? subunits. The researchers adhered the ??3?3 complex to a glass slide so that the ? subunit was protruding upward. Because the ? subunit is too small to be seen with a light microscope, it is not possible to visualize the rotation of the ? subunit directly. To circumvent this problem, the researchers attached a large, fluorescently labelled actin filament to the ? subunit via a linker protein. The fluorescently labelled actin filament is very long compared with the ? subunit and can be readily seen with a fluorescence microscope. Because the membrane-embedded portion of the protein was missing, the researchers added ATP to get the ? subunit to rotate. Although the normal function of the ATP synthase is to make ATP, it can run backward. In other words, the ATP synthase can hydrolyze ATP. As shown in the data for Figure 7.20, when the researchers added ATP, the fluorescently labelled actin filament was observed to rotate counterclockwise, which is opposite to the direction that the ? subunit rotates when ATP is synthesized. In fact, actin filaments were observed to make more than 100 revolutions in the presence of ATP. These results convinced the scientific community that the ATP synthase is indeed a rotary machine. Picture Stats: Views: 1470 Filesize: 137.15kB Height: 1150 Width: 850 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=338 Keywords: Evidence that the ATP synthase is a rotary machine |
