 |
| Previous Image | Next Image |
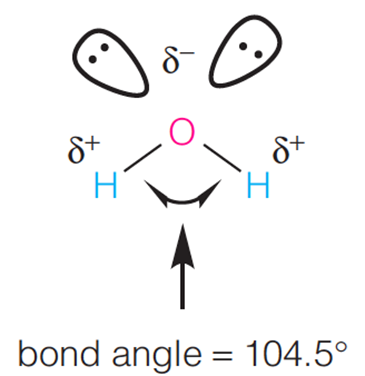
| Description: Hydrogen-bond donors and acceptors in water. The two nonbonded electron pairs on O act as H-bond acceptors and the two O-H bonds act as H-bond donors. The angle between the two O-H bonds is 104.5; thus, water has a net dipole moment. Picture Stats: Views: 407 Filesize: 32.49kB Height: 376 Width: 365 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=33821 |
