 |
| Previous Image | Next Image |
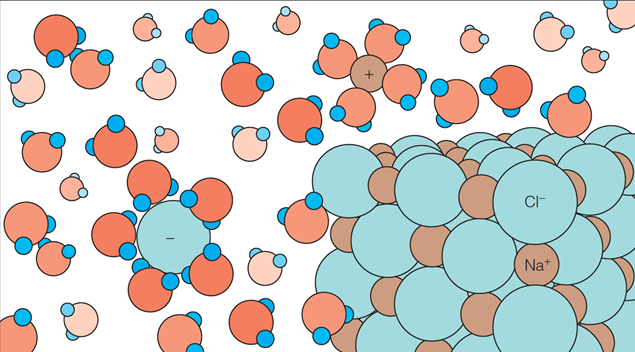
| Description: The Role of Water in Biological Processes Hydration of ions in solution. A salt crystal is shown dissolving in water. As sodium and chloride ions leave the crystal, the noncovalent interaction between these ions and the dipolar water molecules produces a hydration shell around each ion. The energy released in this interaction helps overcome the charge–charge interactions stabilizing the crystal. Picture Stats: Views: 427 Filesize: 134.9kB Height: 352 Width: 635 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=33825 |
