 |
| Previous Image | Next Image |
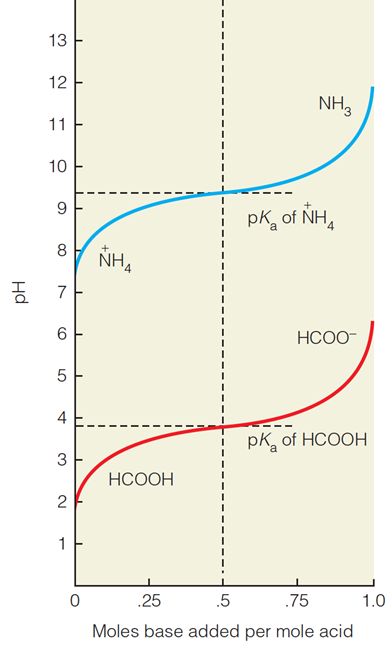
| Description: The Henderson–Hasselbalch equation describes the change in pH during titration of a weak acid or a weak base. Picture Stats: Views: 476 Filesize: 53.44kB Height: 648 Width: 387 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=33832 |
