 |
| Previous Image | Next Image |
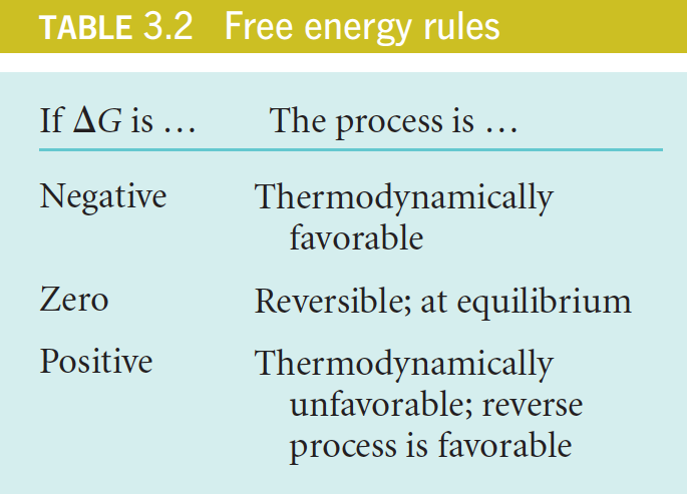
| Description: A thermodynamically favored process tends in the direction that minimizes free energy (results in a negative DG); this is one way of stating the second law of thermodynamics. Processes with a negative free energy changes (-DG) are exergonic Processes with a positive free energy changes (+DG) are endergonic Picture Stats: Views: 389 Filesize: 130.04kB Height: 494 Width: 687 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=33845 |
