 |
| Previous Image | Next Image |
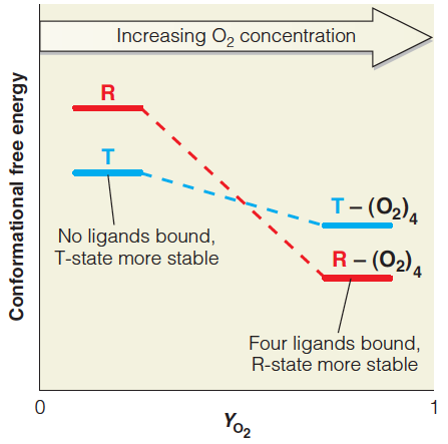
| Description: The deoxy (T) conformation is favored when no ligands are bound, due to the increased number of noncovalent interactions in the T state. As YO2 increases (i.e., more ligands are bound) the energy provided by formation of the Fe-O2 bond stabilizes the R conformation relative to the T conformation. The energetic cost of breaking stabilizing interactions in the deoxy state is paid by the formation of Fe-O2 bonds in the oxy state. Picture Stats: Views: 366 Filesize: 70.54kB Height: 444 Width: 448 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=34125 |
