 |
| Previous Image | Next Image |
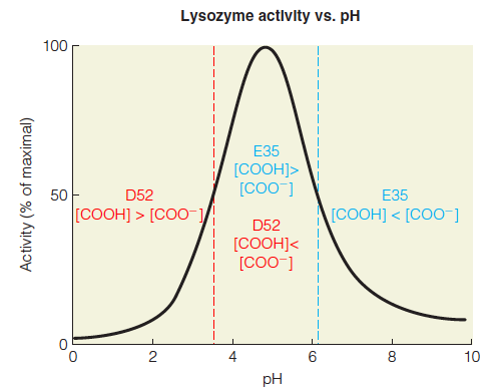
| Description: E35 must be protonated to act as a general acid catalyst in the first step of the mechanism; thus, at pH values below 6.2 the ratio of [COOH]/[COO-] is greatest, favoring catalysis. D52 must be deprotonated to interact with the oxocarbenium ion; thus, at pH values above 3.7 the ratio of [COO-]/[COOH] is greatest, favoring catalysis. These two boundary requirements give rise to the observed pH optimum (~5) where both protonated E35 and deprotonated D52 are abundant. Picture Stats: Views: 406 Filesize: 61.58kB Height: 391 Width: 500 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=34376 |
