 |
| Previous Image | Next Image |
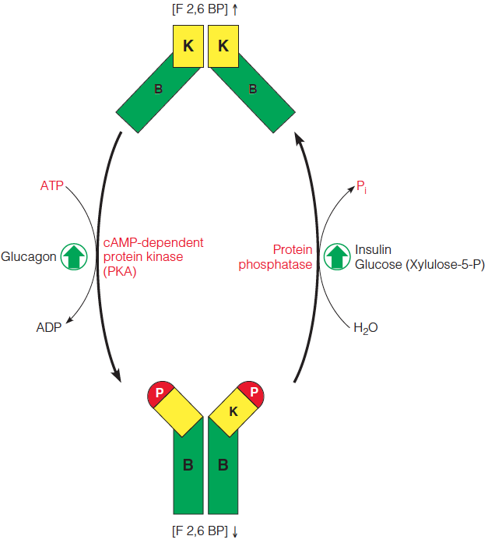
| Description: The bifunctional PFK-2/FBPase-2 is controlled by reversible phosphorylation of a specific serine residue near the N-terminus of each subunit of the homodimeric protein. In the unphosphorylated form, the 6-phosphofructo-2-kinase domain (K) is active, and fructose-2,6-bisphosphate (F2,6BP) is synthesized. In the phosphorylated form, the fructose-2,6-bisphosphatase domain (B) is active, and F2,6BP is degraded. Glucagon stimulates phosphorylation by activating cAMP-dependent protein kinase (PKA). Insulin and glucose (via xyulose-5-phosphate) stimulate dephosphorylation by activating a protein phosphatase. Picture Stats: Views: 312 Filesize: 55.64kB Height: 550 Width: 488 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=34539 |
