 |
| Previous Image | Next Image |
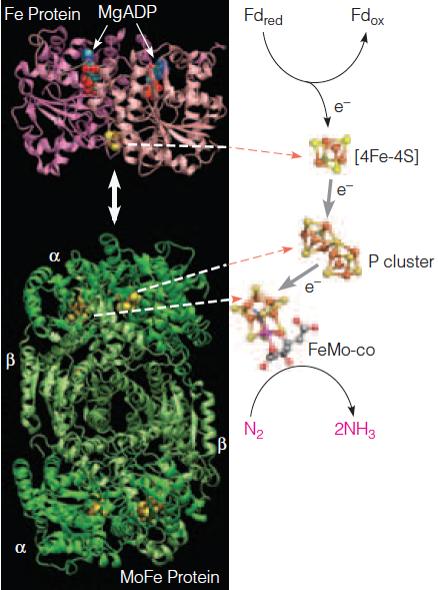
| Description: Left: The two subunits of the homodimeric Fe protein each containing a bound MgADP and the bridging Fe4–S4 cluster. Each unit binds one P iron–sulfur cluster and one FeMo-co iron–sulfur cluster. Right: The relative positions and structures of the Fe4–S4 cluster of the Fe protein, and the P cluster and the FeMo cofactor (FeMo-co) of the MoFe protein are shown. Hydrolysis of bound ATP is thought both to drive the reduction of P cluster by Fe protein and to trigger a conformational change in Fe protein that causes it to dissociate transiently from MoFe protein, assuring unidirectional electron flow. Picture Stats: Views: 319 Filesize: 373.89kB Height: 590 Width: 438 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=34732 |
