 |
| Previous Image | Next Image |
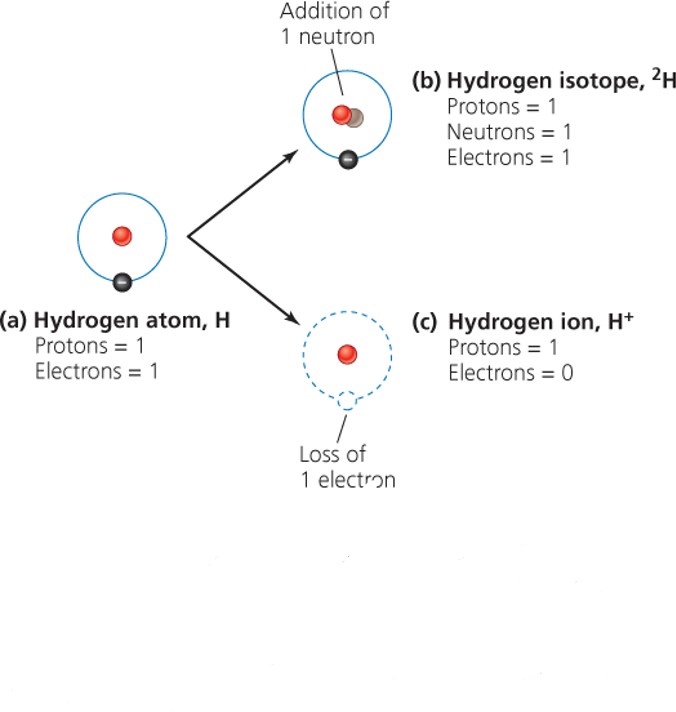
| Description: - Isotopes = atoms with differing numbers of neutrons - Mass number = the combined number of protons and neutrons - - Isotopes of an element behave differently - - Some isotopes are radioactive and decay until they become non-radioactive stable isotopes - - Emit high-energy radiation - Half-life = the amount of time it takes for one-half of the atoms to give off radiation and decay - - Different radioscopes have different half-lives - - Ranging from fractions of a second to billions of years - - Uranium-235: Used in commercial nuclear power and Has a half-life of 700 million years - Atoms may also gain or lose electrons to become ions, electrically charged atoms Picture Stats: Views: 508 Filesize: 223.97kB Height: 712 Width: 676 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=35599 |
