 |
| Previous Image | Next Image |
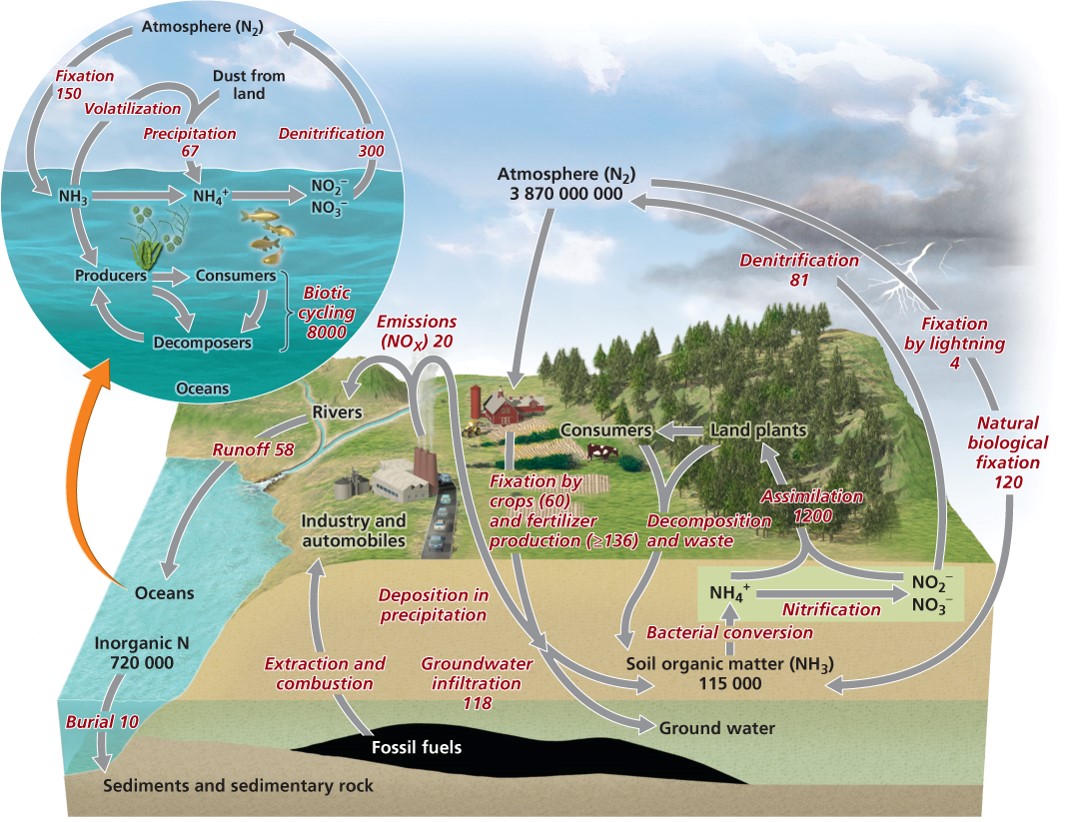
| Description: Nitrogen is 78% of our atmosphere but N2 gas is inert (not a usable form) Nitrogen fixation = Nitrogen gas is fixed (made into ammonia) by nitrogen-fixing bacteria. For example, Usable form (ammonium ions) Nitrification = bacteria that convert ammonium ions first into nitrite ions then into nitrate ions. Plants can take up these ions Animals obtain nitrogen by eating plants or other animals Denitrifying bacteria = convert nitrates in soil or water to gaseous nitrogen, releasing it back into the atmosphere Haber-Bosch process = synthetic production of fertilizers by combining nitrogen and hydrogen to synthesize ammonia Humans are fixing as much nitrogen as nature does BOD = biochemical oxygen demand Reduces dissolved oxygen in water. Lead to hypoxia and Impacts organisms Haber-Bosch process = synthetic production of fertilizers by combining nitrogen and hydrogen to synthesize ammonia Humans are fixing as much nitrogen as nature does BOD = biochemical oxygen demand Reduces dissolved oxygen in water. Lead to hypoxia and Impacts organisms Haber-Bosch process = synthetic production of fertilizers by combining nitrogen and hydrogen to synthesize ammonia Humans are fixing as much nitrogen as nature does BOD = biochemical oxygen demand Reduces dissolved oxygen in water. Lead to hypoxia and Impacts organisms Haber-Bosch process = synthetic production of fertilizers by combining nitrogen and hydrogen to synthesize ammonia Humans are fixing as much nitrogen as nature does Picture Stats: Views: 291 Filesize: 221.62kB Height: 822 Width: 1071 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=35636 |
