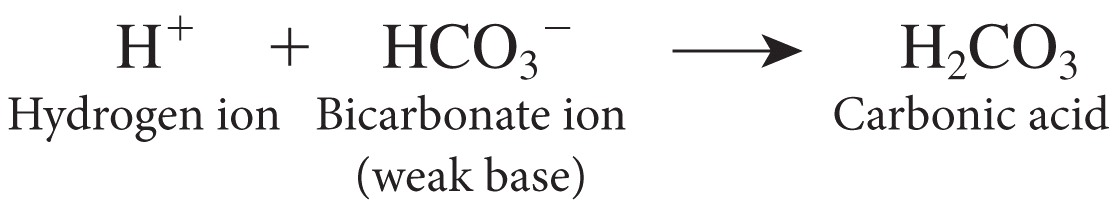 |
| Previous Image | Next Image |
| Description: Carbonic acid-bicarbonate buffer system: this is based on the bicarbonate ion (HCO3–) which acts as a weak base, and carbonic acid (H2CO3) which acts as a weak acid. If the pH falls, the HCO3– removes excess H+: Picture Stats: Views: 1116 Filesize: 69.83kB Height: 223 Width: 1119 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=40388 |
