 |
| Previous Image | Next Image |
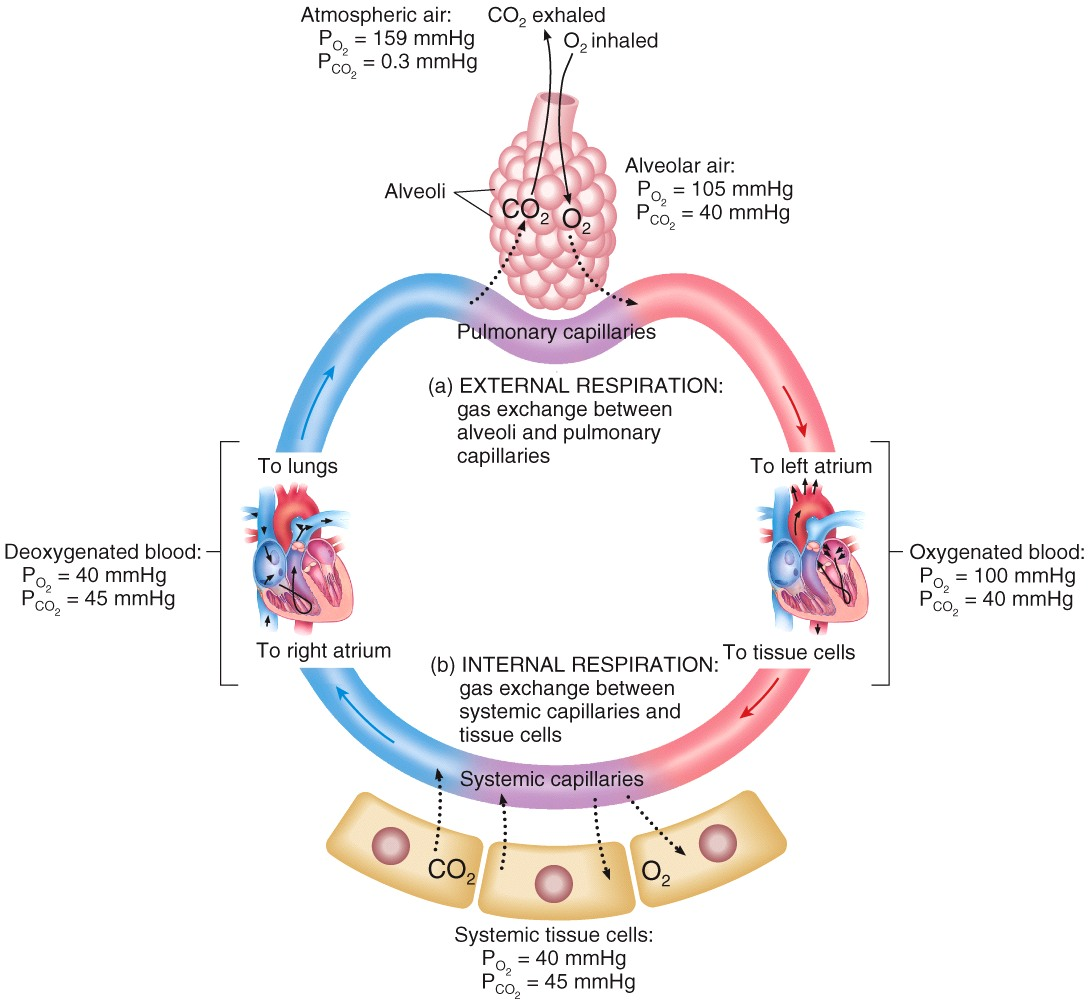
| Description: Dalton’s law Each gas in a mixture of gases exerts its own pressure as if no other gases were present Henry’s law The quantity of a gas that will dissolve in a liquid is proportional to the partial pressure of the gas and its solubility coefficient when the temperature remains constant External and Internal Respiration During external respiration, oxygen will diffuse from the alveoli into the pulmonary capillaries CO2 moves in the opposite direction During internal respiration, oxygen will diffuse from the systemic capillaries into the tissue CO2 moves in the opposite direction Picture Stats: Views: 377 Filesize: 617.14kB Height: 1000 Width: 1092 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=40822 |
