 |
| Previous Image | Next Image |
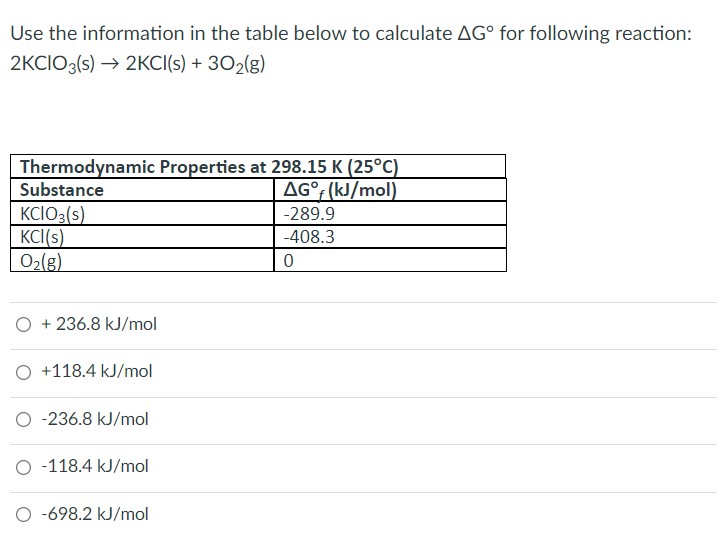
| Description: Use the information in the table below to calculate ΔG° for following reaction: 2KClO3(s) → 2KCl(s) + 3O2(g) + 236.8 kJ/mol +118.4 kJ/mol -236.8 kJ/mol -118.4 kJ/mol -698.2 kJ/mol Use the information in the table below to calculate A6” for foliowing reaction: 2KCiOg(5) ~> 2KC|(Si + 302%) Thermodynamic Properties at 293,15 K (25°C) Substance AG°,(kJ/mo|) KCiOgis) 7289.9 KCi(s) 7403 3 02(3! 0 Q + 236.8 kJ/mol () +1184 kJ/moi C 436.8 kJ/moi Q 7118.4 kJ/moi Q 1398.2 kJ/moi Picture Stats: Views: 341 Filesize: 54.49kB Height: 553 Width: 717 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=44646 |
