 |
| Previous Image | Next Image |
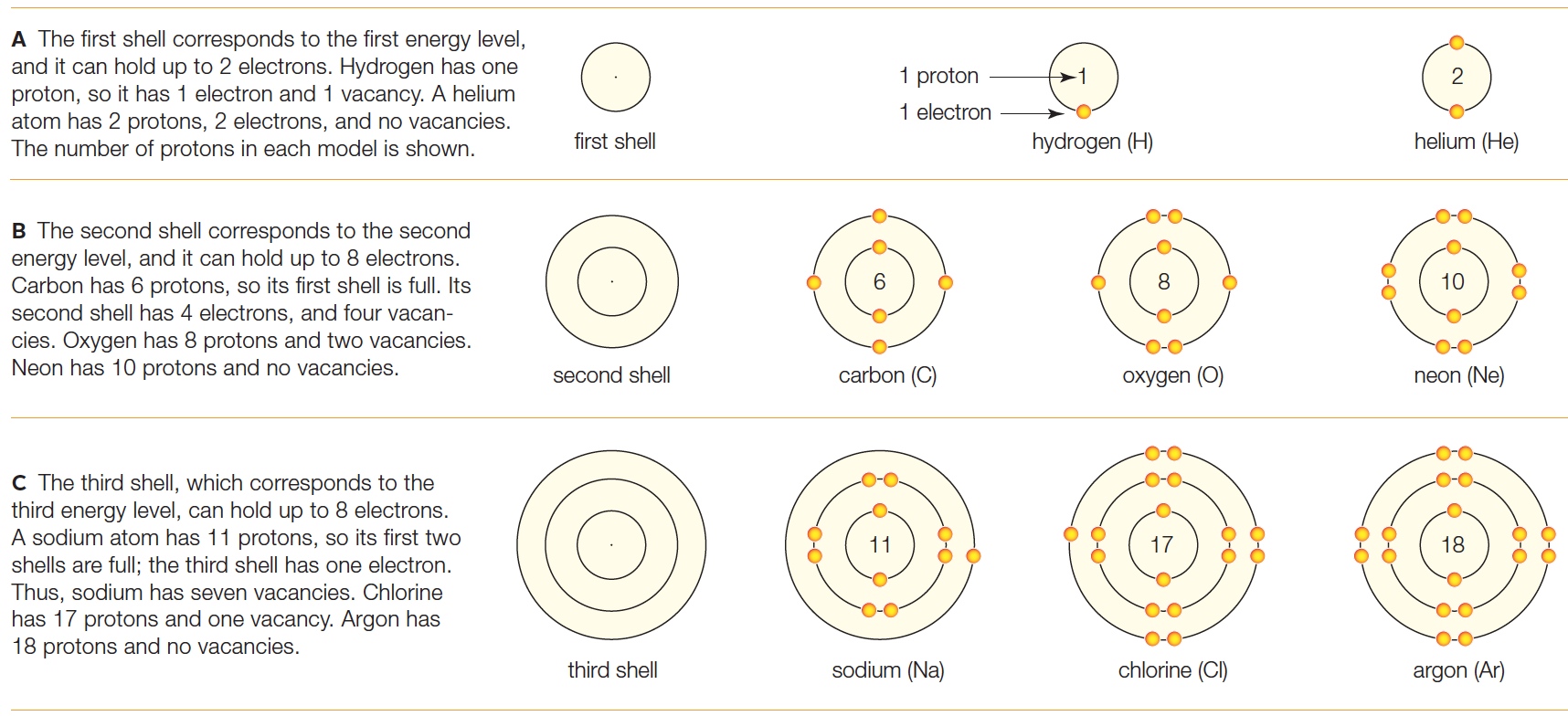
| Description: Each circle (shell) represents all orbitals at one energy level. A model is filled with electrons from the innermost shell out, until there are as many electrons as protons. Atoms with vacancies (room for additional electrons) in their outermost shell tend to get rid of them. A The first shell corresponds to the first energy level, and it can hold up to 2 electrons. Hydrogen has one proton, so it has 1 electron and 1 vacancy. A helium atom has 2 protons, 2 electrons, and no vacancies. The number of protons in each model is shown. B The second shell corresponds to the second energy level, and it can hold up to 8 electrons. Carbon has 6 protons, so its first shell is full. Its second shell has 4 electrons, and four vacancies. Oxygen has 8 protons and two vacancies. Neon has 10 protons and no vacancies. B The second shell corresponds to the second energy level, and it can hold up to 8 electrons. Carbon has 6 protons, so its first shell is full. Its second shell has 4 electrons, and four vacancies. Oxygen has 8 protons and two vacancies. Neon has 10 protons and no vacancies. A The first shell corresponds to the first energy level, and it can hold up to 2 electrons. Hydrogen has one . . O 1 proton 461 2) proton, so It has 1 electron and 1 vacancy. A helium atom has 2 protons, 2 electrons, and no vacancies. 1 electron 4’ . . The number of protons in each model is shown. first shell hydrogen (H) helium (He) _. _ B The second shell corresponds to the second energy level, and it can hold up to 8 electrons. . f. \' Carbon has 6 protons, so its first shell is full. Its <  . (10> . second shell has 4 electrons, and four vacan» . o / cies. Oxygen has 8 protons and two vacancies. ._. _. Neon has 10 protons and no vacancies. second shell carbon (C) oxygen (0) neon (Ne) 0‘. 0'. C The third shell, which corresponds to the ’0 0’ third energy level, can hold up to 8 electrons. /.O \ / 0.\ A sodium atom has 11 protons, so its first two 0 ,0 17 9, 0, ,0 ,0 18 0, 9 shells are full; the third shell has one electron. . j . . .\ ‘j . , . . O 0 Thus, sodium has seven vacancies. Chlorine K. has 17 protons and one vacancy. Argon has "/ \" / 18 protons and no vacancies. .'. .'. third shell sodium (Na) chlorine (Cl) argon (A) . (10> . second shell has 4 electrons, and four vacan» . o / cies. Oxygen has 8 protons and two vacancies. ._. _. Neon has 10 protons and no vacancies. second shell carbon (C) oxygen (0) neon (Ne) 0‘. 0'. C The third shell, which corresponds to the ’0 0’ third energy level, can hold up to 8 electrons. /.O \ / 0.\ A sodium atom has 11 protons, so its first two 0 ,0 17 9, 0, ,0 ,0 18 0, 9 shells are full; the third shell has one electron. . j . . .\ ‘j . , . . O 0 Thus, sodium has seven vacancies. Chlorine K. has 17 protons and one vacancy. Argon has "/ \" / 18 protons and no vacancies. .'. .'. third shell sodium (Na) chlorine (Cl) argon (A)
Picture Stats: Views: 690 Filesize: 419.83kB Height: 784 Width: 1716 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=47160 |
