 |
| Previous Image | Next Image |
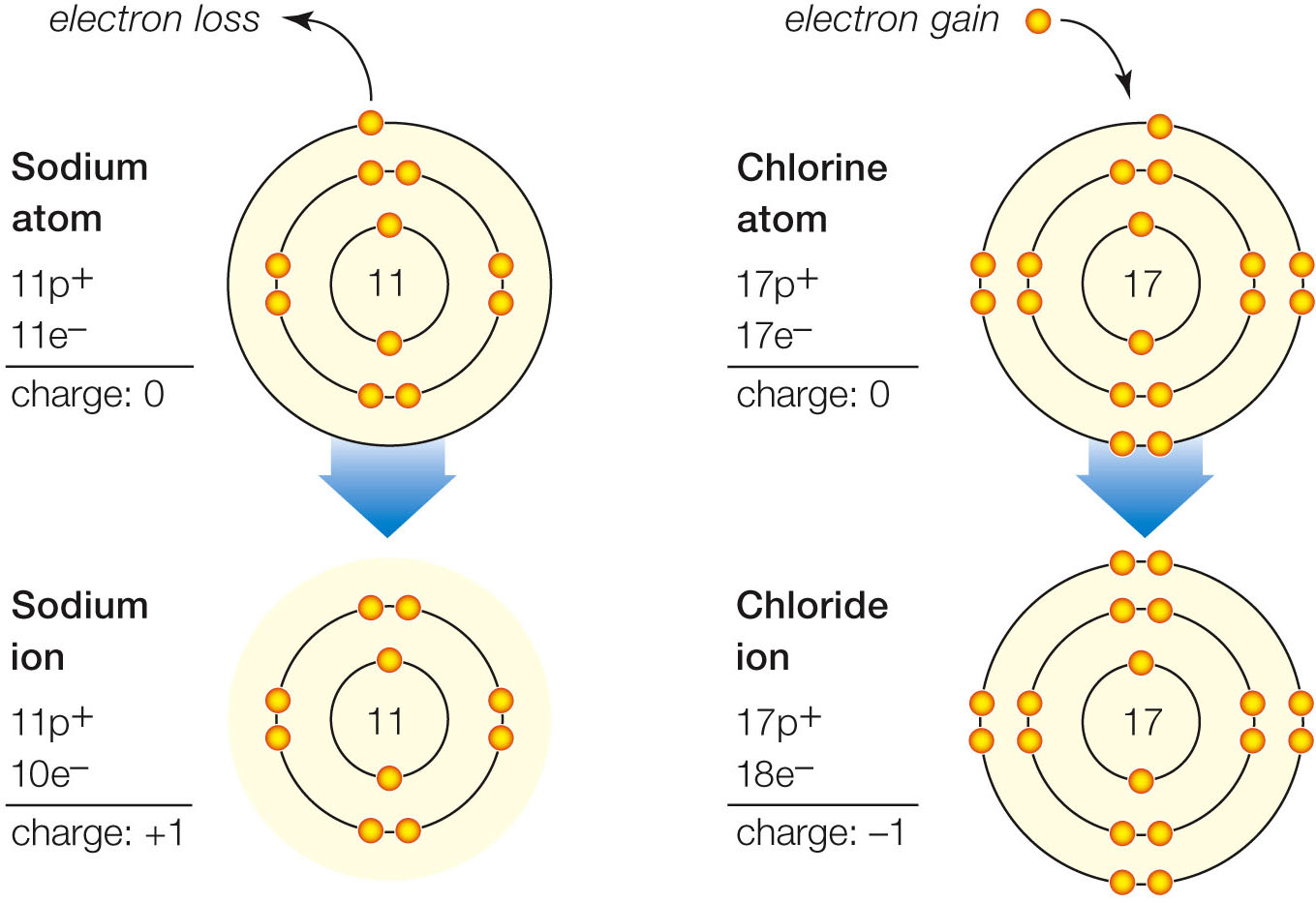
| Description: A A sodium atom (Na) becomes a positively charged sodium ion (Na+) when it loses the electron in its third shell. The atom’s full second shell is now its outermost, so it has no vacancies. B A chlorine atom (Cl) becomes a negatively charged chloride ion (Cl–) when it gains an electron and fills the vacancy in its third, outermost shell. electron loss Sodium atom 11p+ 11e— chage:0 Sodium ion 11p+ 10e— chage:+1 electron gain 0 Chlorine .— atom ./ 17p+ : I. < 17e— \ charge: 0 Chloride ion / 18e- \ charge: —1 Picture Stats: Views: 696 Filesize: 149.14kB Height: 933 Width: 1359 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=47161 |
