 |
| Previous Image | Next Image |
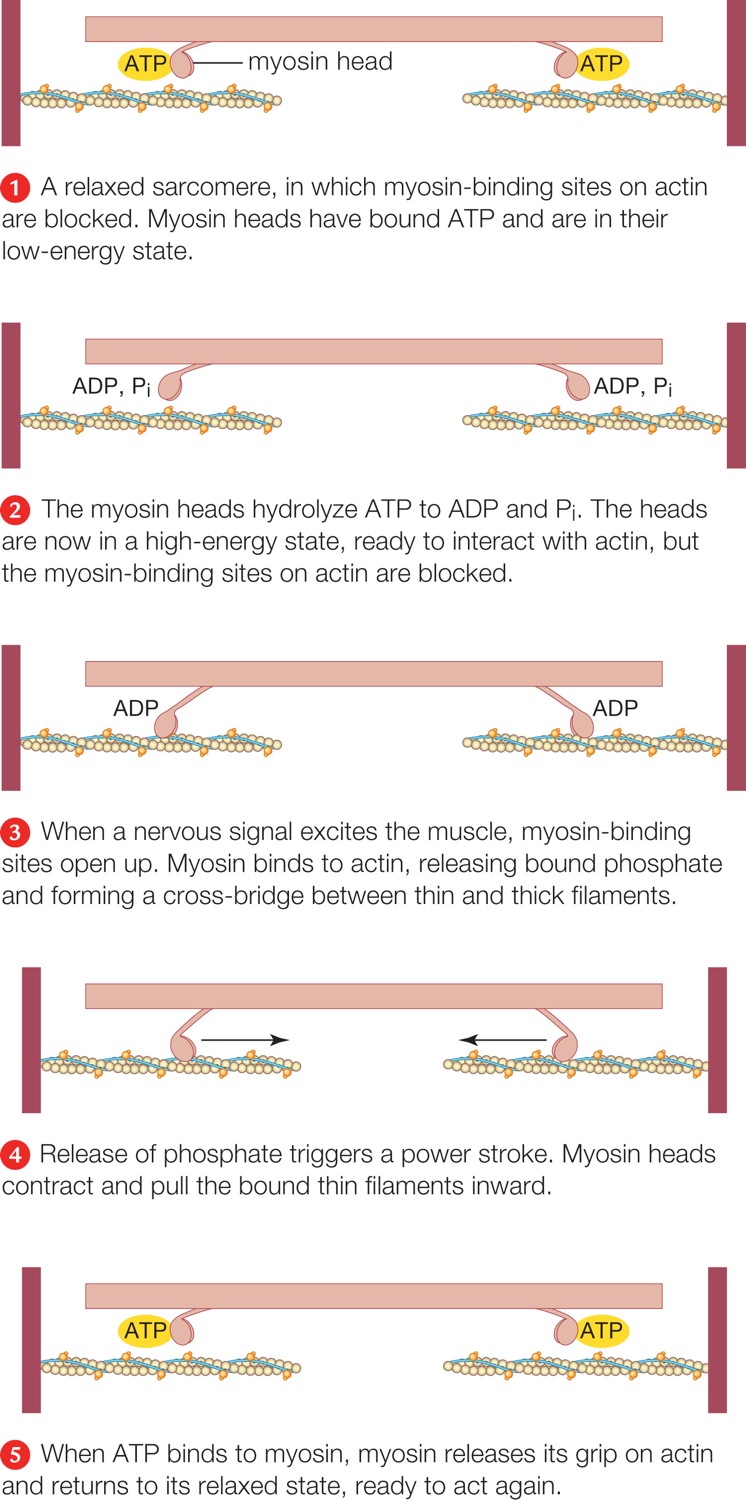
| Description: Above, during muscle contraction, thin filaments slide inward past thick filaments, reducing the width of the sarcomere.Right, molecular mechanism of contraction. For clarity, we show only two myosin heads. A head binds repeatedly to an actin filament and slides it toward the center of the sarcomere. A relaxed sarcomere, in which myosin-binding sites on actin are blocked. Myosin heads have bound ATP and are in their low-energy state. The myosin heads hydrolyze ATP to ADP and Pi. The heads are now in a high-energy state, ready to interact with actin, but the myosin-binding sites on actin are blocked. When a nervous signal excites the muscle, myosin-binding sites open up. Myosin binds to actin, releasing bound phosphate and forming a cross-bridge between thin and thick filaments. Release of phosphate triggers a power stroke. Myosin heads contract and pull the bound thin filaments inward. When ATP binds to myosin, myosin releases its grip on actin and returns to its relaxed state, ready to act again. Picture Stats: Views: 857 Filesize: 249.54kB Height: 1500 Width: 746 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=47612 |
