 |
| Previous Image | Next Image |
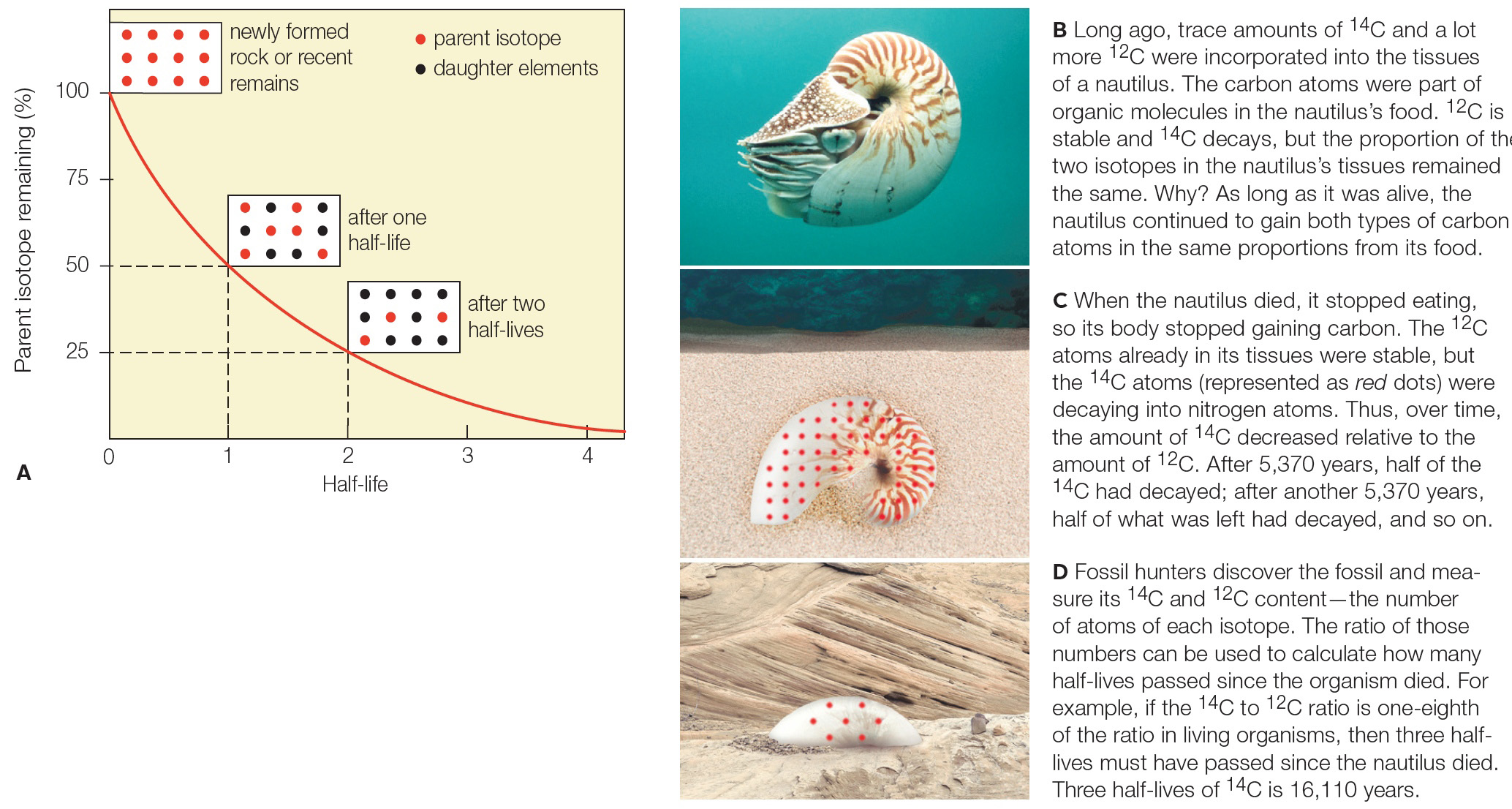
| Description: Long ago, trace amounts of 14C and a lot more 12C were incorporated into the tissues of a nautilus. The carbon atoms were part of organic molecules in the nautilus’s food. 12C is stable and 14C decays, but the proportion of the two isotopes in the nautilus’s tissues remained the same. Why? As long as it was alive, the nautilus continued to gain both types of carbon atoms in the same proportions from its food. When the nautilus died, it stopped eating, so its body stopped gaining carbon. The 12C atoms already in its tissues were stable, but the 14C atoms (represented as red dots) were decaying into nitrogen atoms. Thus, over time, the amount of 14C decreased relative to the amount of 12C. After 5,370 years, half of the 14C had decayed; after another 5,370 years, half of what was left had decayed, and so on. Fossil hunters discover the fossil and measure its 14C and 12C content—the number of atoms of each isotope. The ratio of those numbers can be used to calculate how many half-lives passed since the organism died. For example, if the 14C to 12C ratio is one-eighth of the ratio in living organisms, then three half-lives must have passed since the nautilus died. Three half-lives of 14C is 16,110 years. Picture Stats: Views: 699 Filesize: 571.35kB Height: 1106 Width: 2068 Source: https://biology-forums.com/index.php?action=gallery;sa=view;id=48576 |
