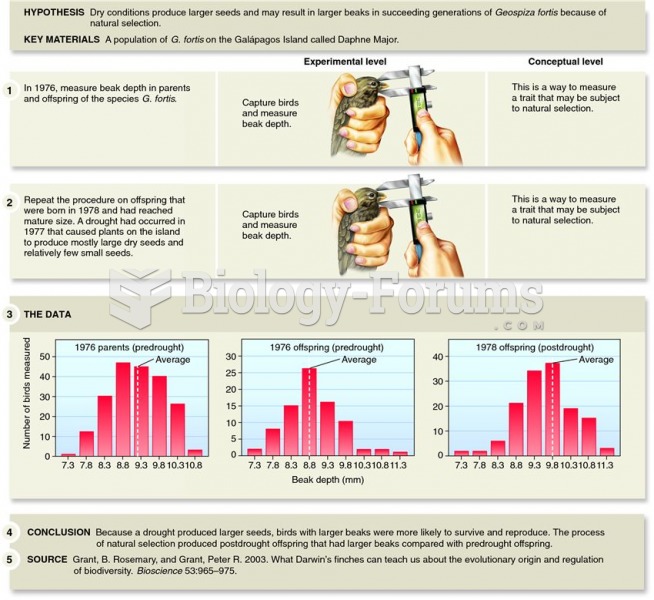
Answer to Question 1
Answer: The drag force equals the gravitation force, causing droplets to fall at their terminal velocity. They usually evaporate before reaching the ground.
Answer to Question 2
Answer: The principle underlying the Bergeron process is that the saturation vapor pressure over ice (the amount of water vapor needed to keep it in equilibrium) is less than that over supercooled water at the same temperature. This is because molecules in an ice crystal bond to each other more tightly than molecules of liquid water. You should recall that saturation exists when the vapor pressure of the air is at the point where evaporation from a water droplet would be exactly offset by condensation back onto it, or if sublimation from an ice crystal would be offset by deposition. Within a certain range of temperatures below 0 C, both ice crystals and supercooled droplets can coexist in a cloud. Ice crystals, however, do not sublimate ice to vapor as rapidly as water droplets evaporate liquid to vapor. Thus, ice crystals do not require as high a surrounding vapor pressure as do water droplets to remain in equilibrium, and they are said to have a lower saturation vapor pressure. As a result, if there is just enough water vapor in the air to keep a supercooled droplet from evaporating away, then there is more than enough water vapor to maintain an ice crystal. Let us see how that leads to precipitation. Evaporation from the droplet increases the water vapor content of the air, which causes further deposition onto the ice crystals (c). This leads to a continuous transfer in which the liquid droplets surrender water vapor, which is subsequently deposited onto the ice crystals. In other words, the ice crystals continually grow at the expense of the supercooled droplets. Although Figure 76 suggests this process involves distinct steps, evaporation and deposition actually occur simultaneously. The growth of ice crystals by the deposition of water vapor initiates precipitation. As the ice crystals grow, their increasing mass enables them to fall through the cloud and collide with droplets and other ice crystals. The collisions cause two other important processes to occur that greatly accelerate the growth rate of the ice crystals: riming and aggregation.