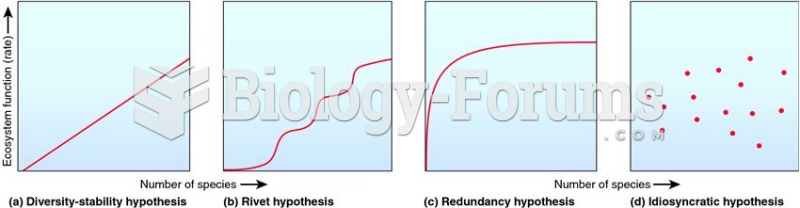
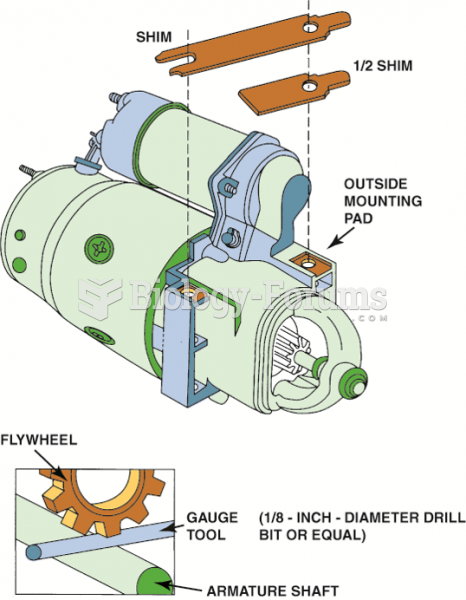
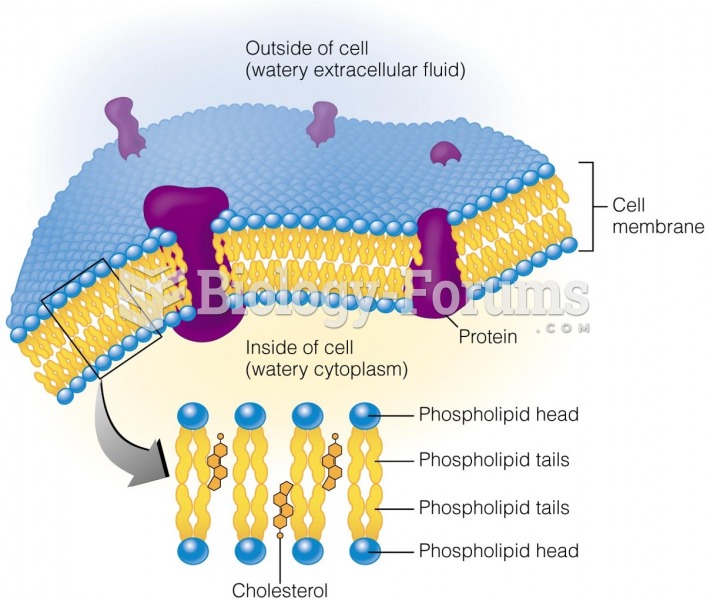
Answer to Question 1
Restoration efforts are needed to repair damage to specific lands and waters so that normal ecosystem integrity, resilience, and productivity return. The ecological problems that can be ameliorated by restoration include those resulting from soil erosion, surface strip mining, draining wetlands, coastal damage, agricultural use, deforestation, overgrazing, desertification, and the eutrophication of lakes. The plan calls for removing 240 miles of levees and canals and creating a system of reservoirs and underground wells to capture water for release during the dry season. The new flowage is designed to restore the river of grass, thereby restoring the 2.4 million acres of Everglades not to original state, but at least to a healthy system.
Answer to Question 2
In 1958, Charles Keeling began measuring CO2 levels on Mauna Loa, in Hawaii. Measurements there have been recorded continuously, and they reveal a striking increase in atmospheric levels of the gas. The concentrations increased exponentially until the energy crisis in the mid-1970s, rose at a rate of 1.5 ppm/year for several decades, and recently began rising at a rate of 1.8 ppm/year and higher. The data also reveal an annual oscillation of 5-7 ppm, which reflects seasonal changes of photosynthesis and respiration of terrestrial ecosystems in the Northern Hemisphere. As of early 2007, atmospheric CO2 levels were more than 380 ppm, 35 higher than they were before the Industrial Revolution and higher than they have been for over 400,000 years.
Significant sources: Every kilogram of fossil fuel burned produces about 3 kg of CO2 that enters the atmosphere. (The mass triples because each carbon atom in the fuel picks up two oxygen atoms in the course of burning and becomes CO2.) Currently, 7.2 million metric tons (gigatons, or Gt) of fossil fuel carbon (GtC) are burned each year, all added to the atmosphere as CO2 (about 3 of this total comes from cement production and is usually included in figures reported for fossil-fuel emissions). At least half of this amount comes from the industrialized countries. It is estimated that the burning of forest trees is another anthropogenic source that adds some 1.6 GtC annually to the carbon already coming from fossil fuel combustion.
Sinks: Careful calculations show that if all the CO2 emitted from burning fossil fuels accumulated in the atmosphere, the concentration would rise by at least 3 ppm per year, not the 2 ppm or less. In the 1990s, this meant an addition of approximately 8 GtC per year added to the atmosphere by anthropogenic sources, yet only 3.3 GtC per year actually accumulated. Thus, there must be carbon sinks' that absorb CO2 and keep it from accumulating at a more rapid rate in the atmosphere. Recent work with stable carbon and oxygen isotopes and careful measurements of atmospheric CO2 around the globe have brought us much closer to quantifying annual fluxes in CO2 and identifying the missing sinks. There is broad agreement that the oceans serve as a sink for much of the CO2 emitted; some of this is due to the uptake of CO2 by phytoplankton and its subsequent sinking, and some is a consequence of the under-saturation of CO2 in seawater. There are limits to the ocean's ability to absorb CO2, however, because only the top 300 m of the ocean is in contact with the atmosphere. Calculations indicate that, in the 1990s, the ocean sink accounted for an uptake of 2.2 + 0.4 GtC annually. Measurements indicate that terrestrial ecosystems can also serve as a carbon sink. Indeed, these land ecosystems apparently stored a net 1.0 + .5 GTC annually during the 1990s, a figure that includes the losses from deforestation and thus implies an average gross annual uptake of some 2.5 GtC during those years. For this reason, terrestrial ecosystems, and especially forests, are increasingly valued because of their ability to sequester carbon. Much of this carbon uptake is being attributed to increased rainfall associated with the warming trend in temperature.