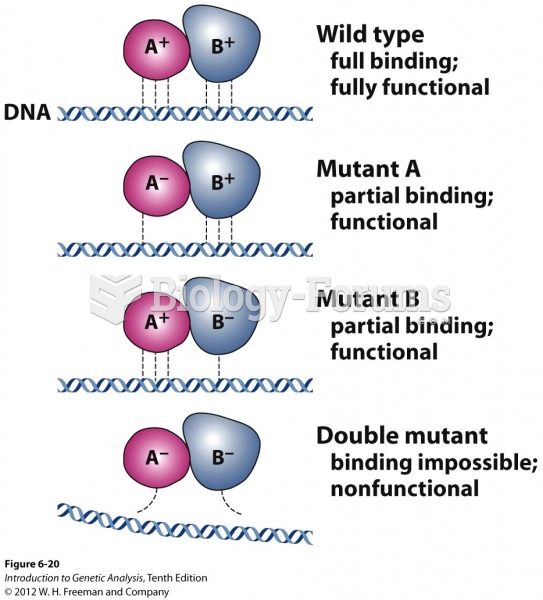
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
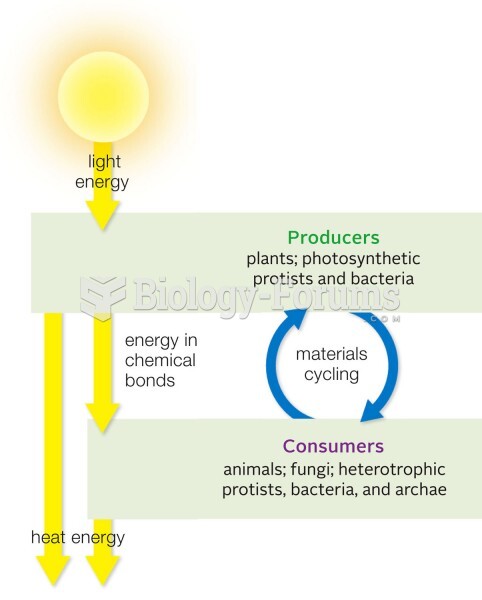
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
Ether was used widely for surgeries but became less popular because of its flammability and its tendency to cause vomiting. In England, it was quickly replaced by chloroform, but this agent caused many deaths and lost popularity.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.