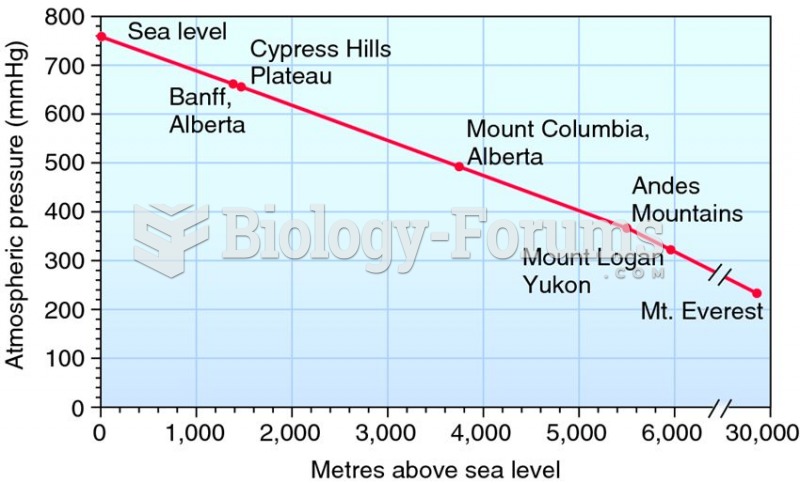
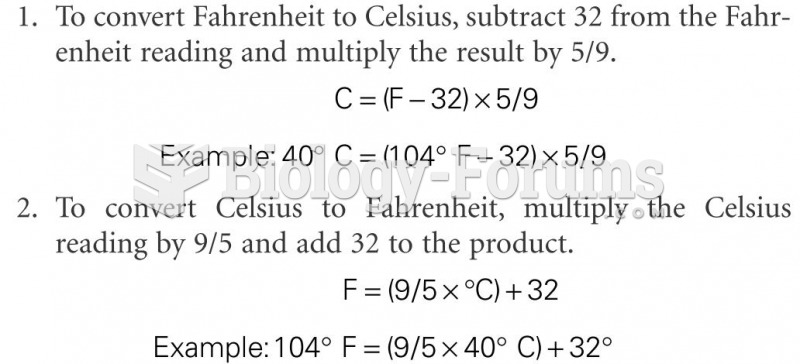
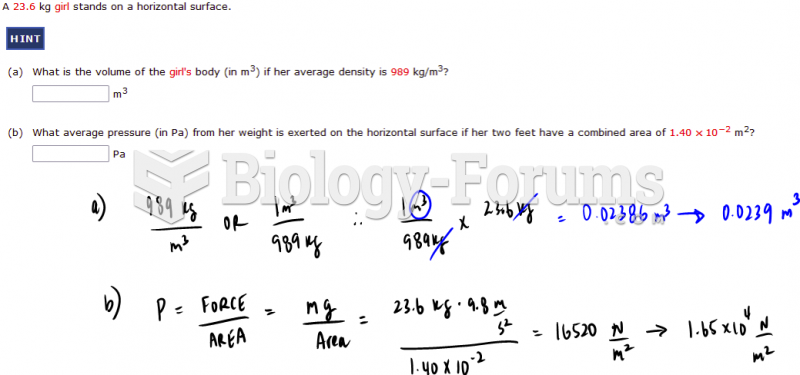
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.