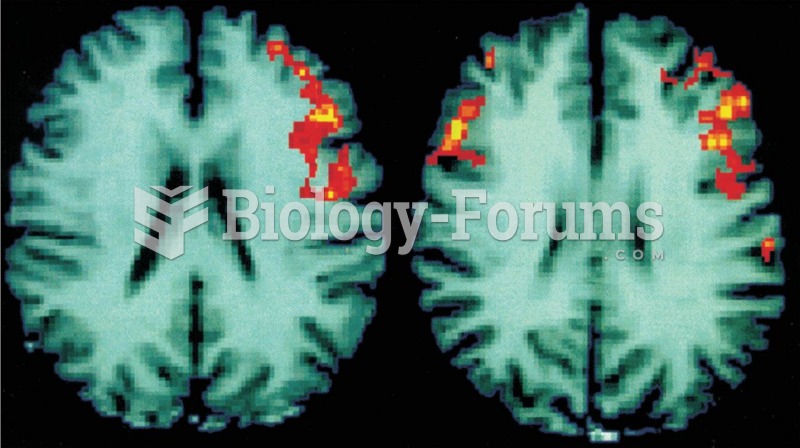
|
|
|
For high blood pressure (hypertension), a new class of drug, called a vasopeptidase blocker (inhibitor), has been developed. It decreases blood pressure by simultaneously dilating the peripheral arteries and increasing the body's loss of salt.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
The average adult has about 21 square feet of skin.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Intradermal injections are somewhat difficult to correctly administer because the skin layers are so thin that it is easy to accidentally punch through to the deeper subcutaneous layer.