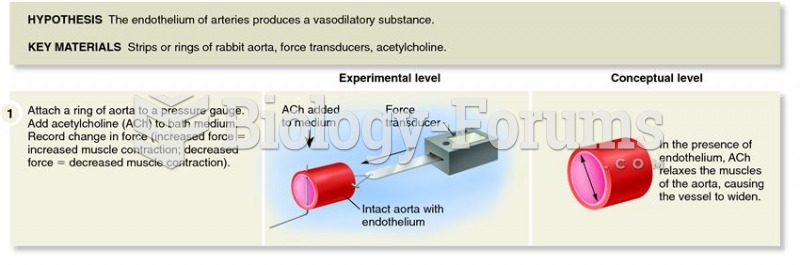
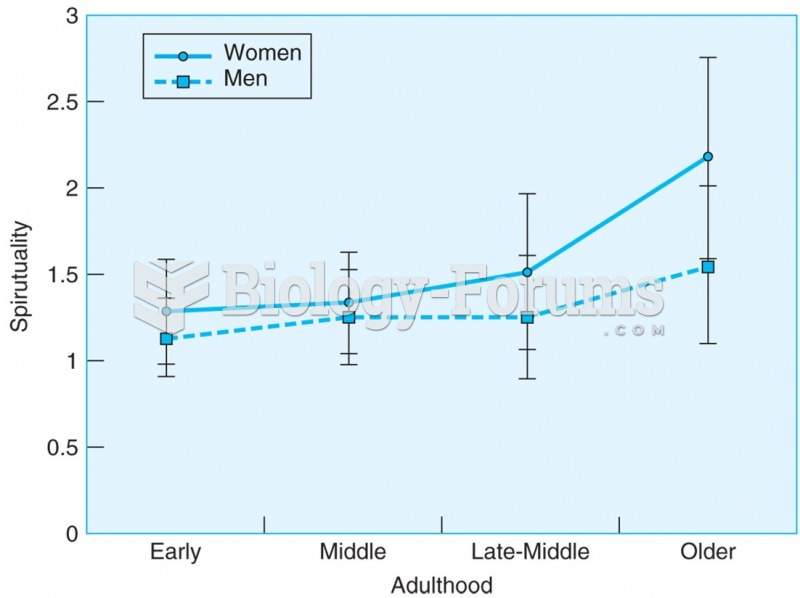
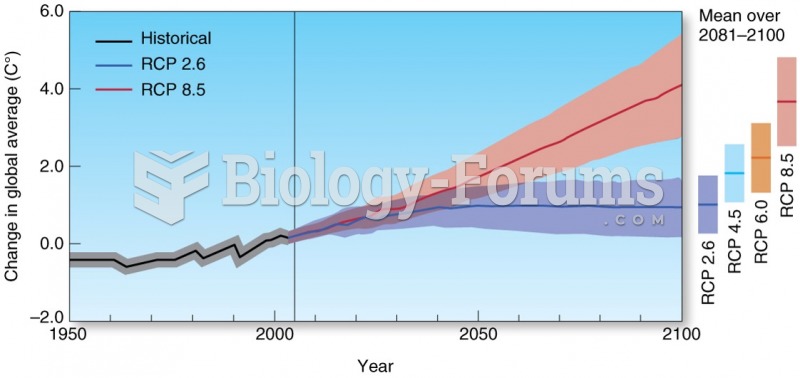
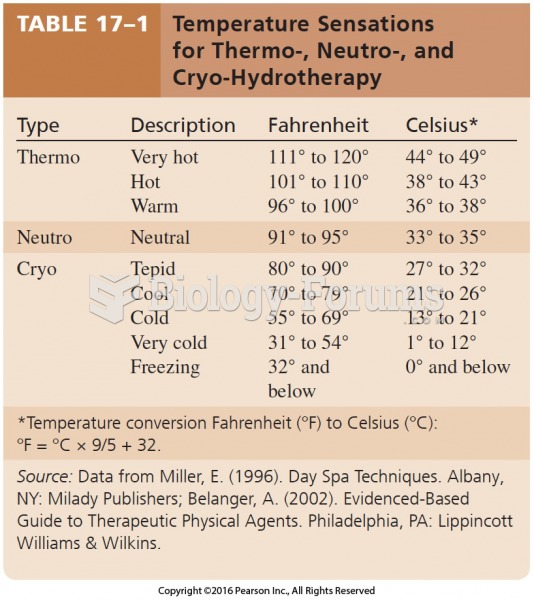
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma occurs in one in 11 children and in one in 12 adults. African Americans and Latinos have a higher risk for developing asthma than other groups.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.