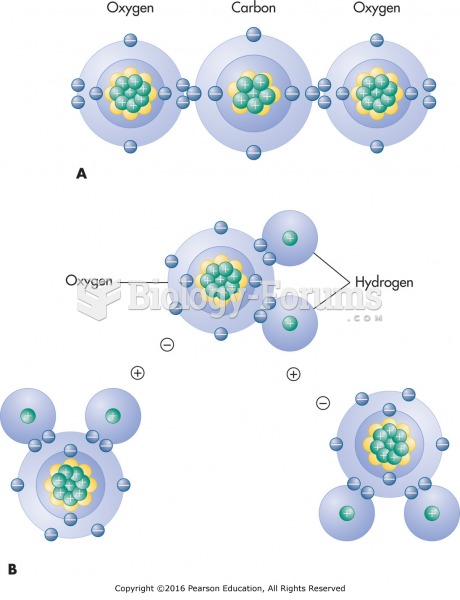
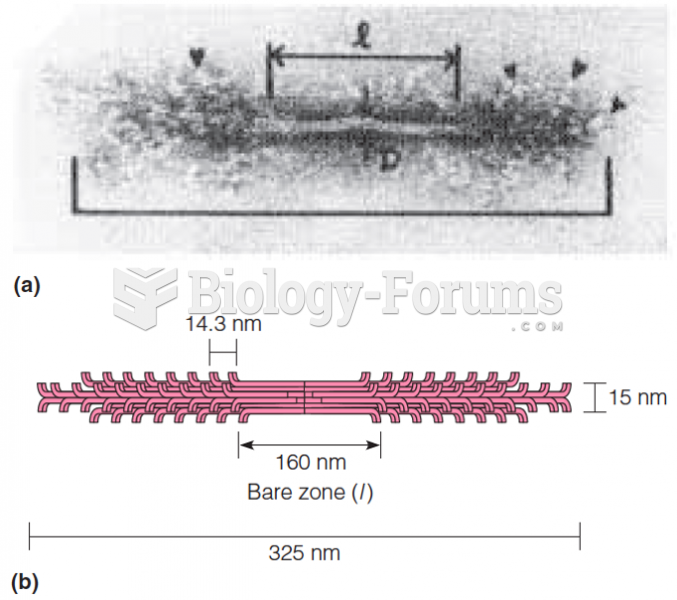
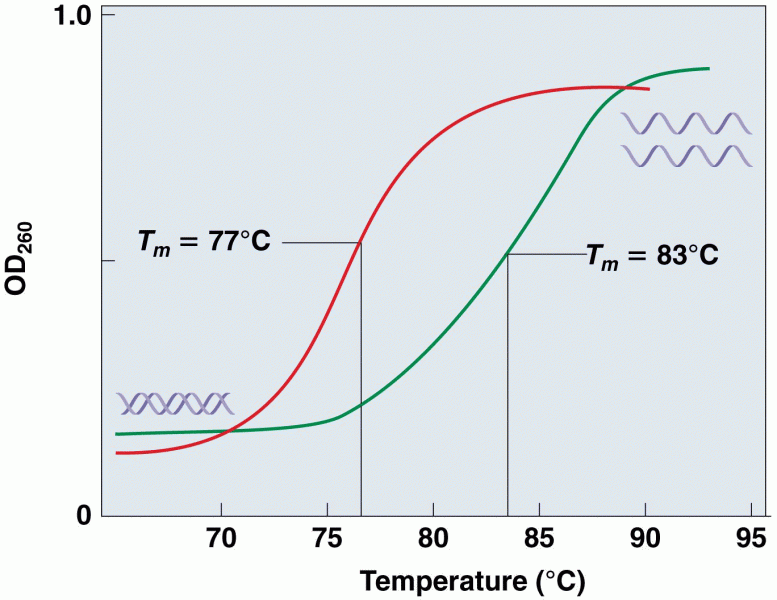
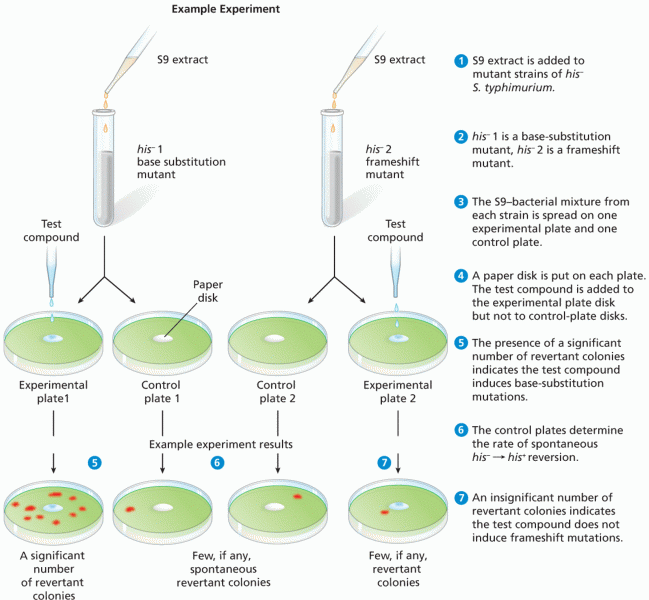
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Your heart beats over 36 million times a year.
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.