|
|
|
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
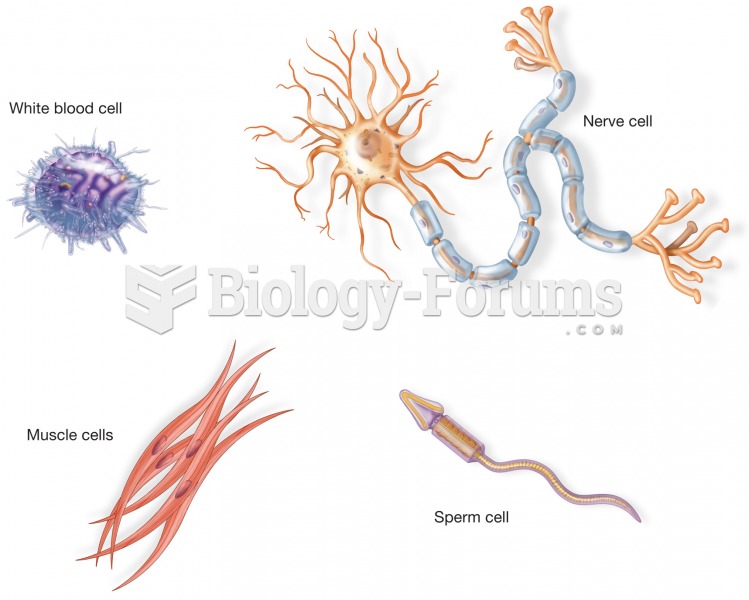 Examples of four different types of cells from the body. Although each cell has a cell membrane, nuc
Examples of four different types of cells from the body. Although each cell has a cell membrane, nuc
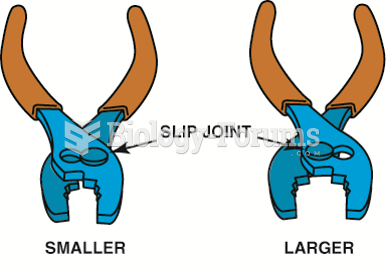 Typical slip-joint pliers are common household pliers. The slip joint allows the jaws to be opened ...
Typical slip-joint pliers are common household pliers. The slip joint allows the jaws to be opened ...