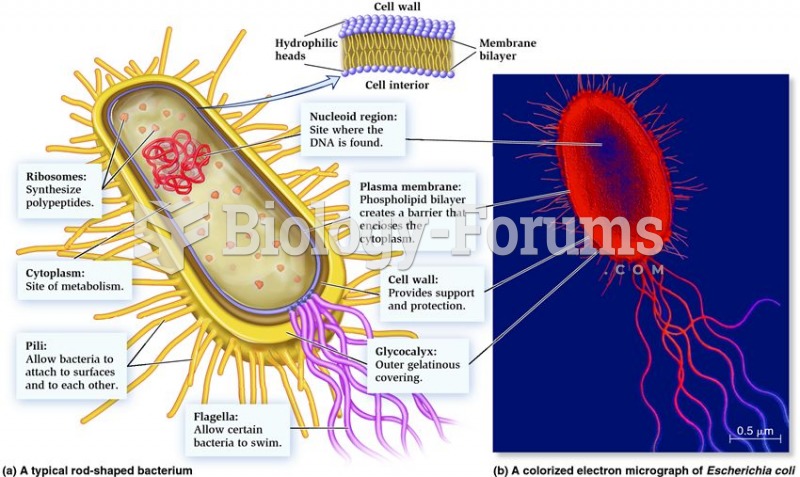
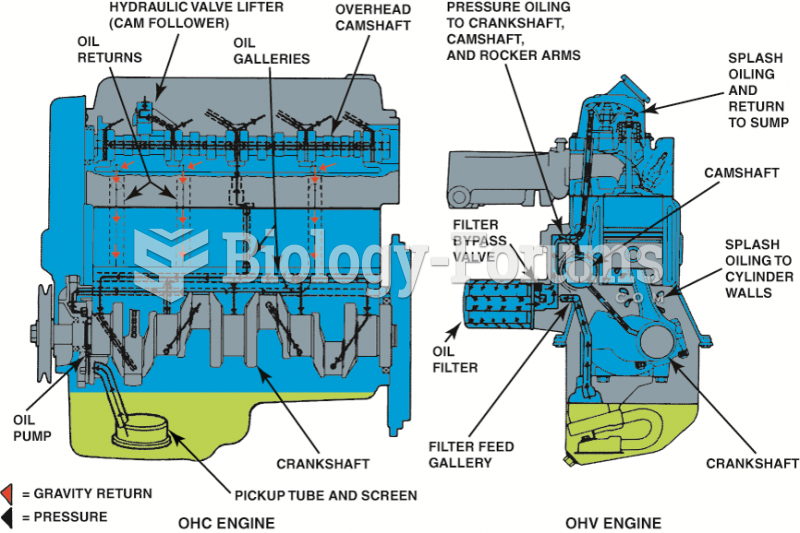
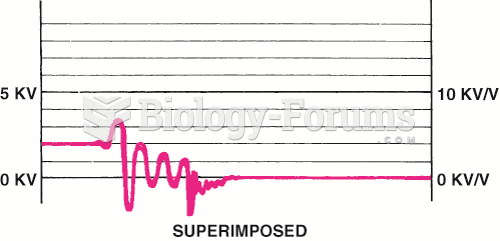
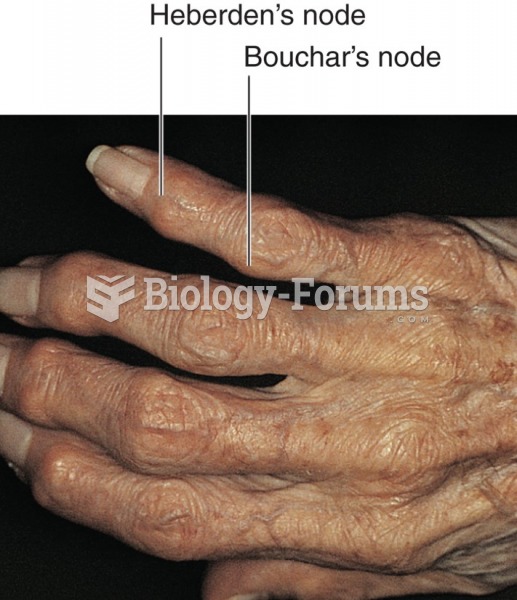
|
|
|
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%