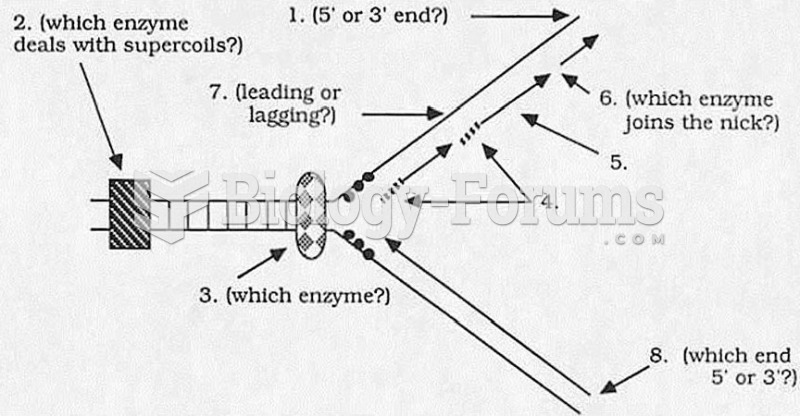
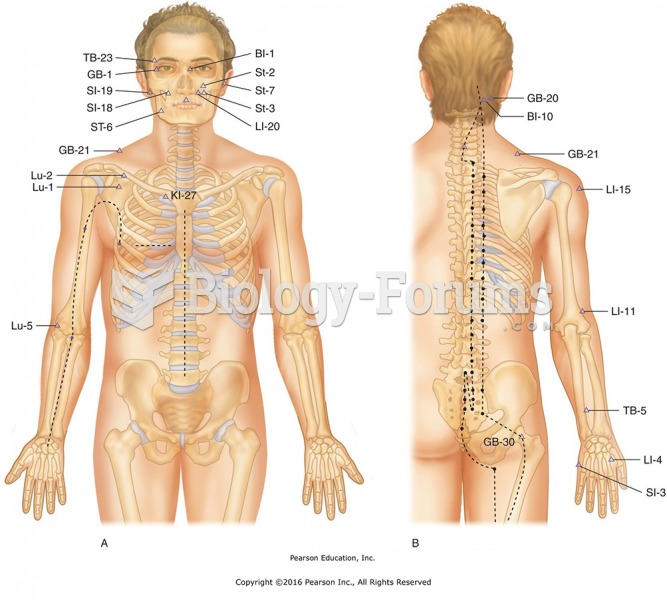
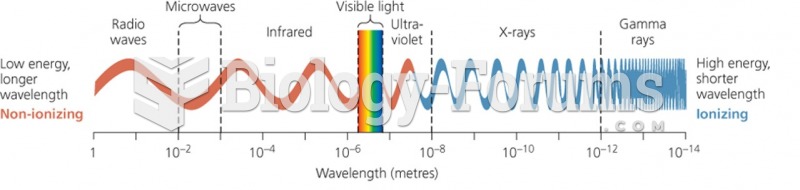
|
|
|
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.