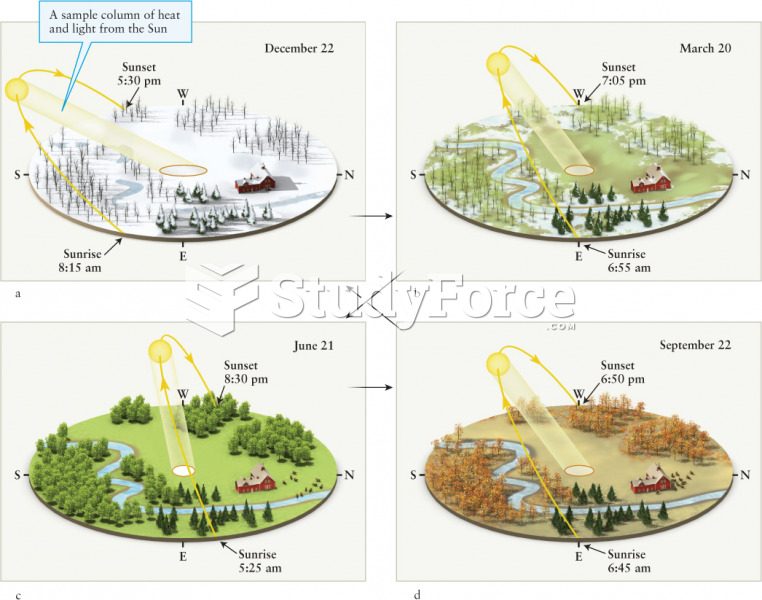
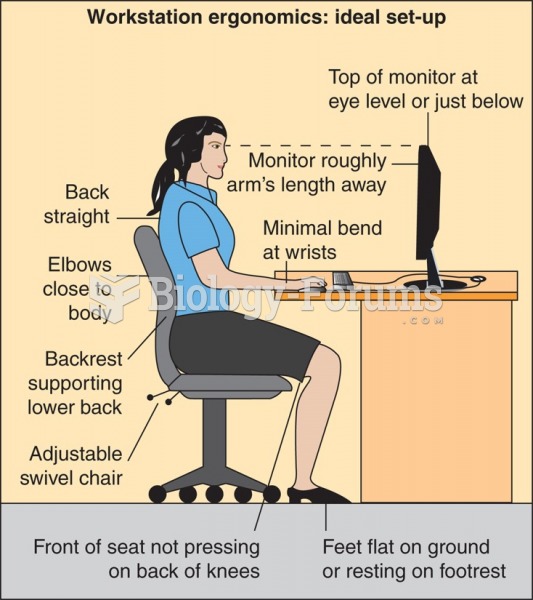
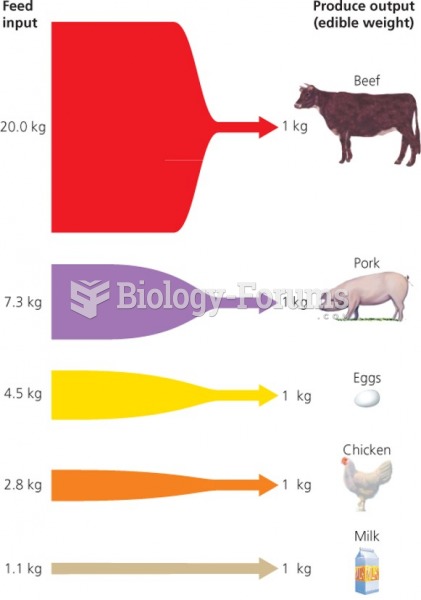
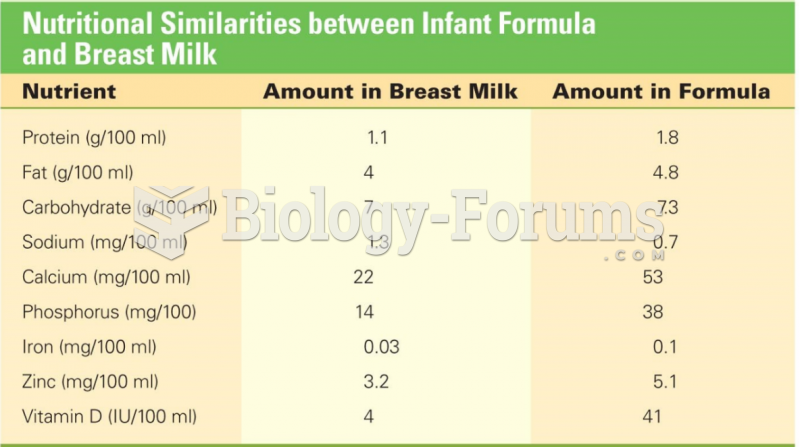
|
|
|
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.