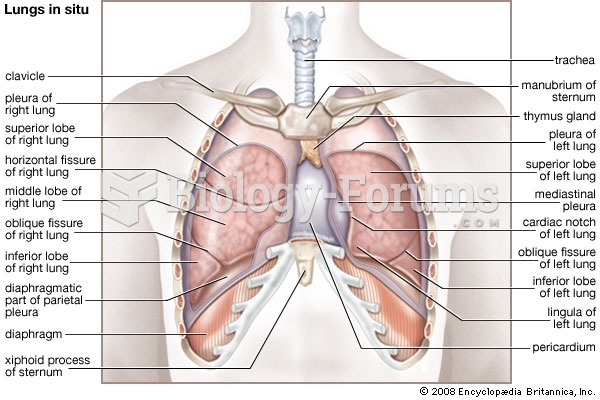
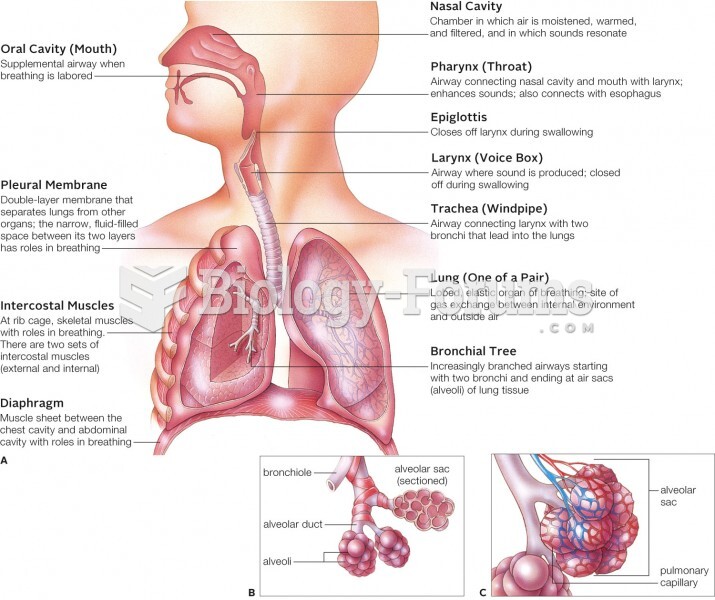
|
|
|
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Children of people with alcoholism are more inclined to drink alcohol or use hard drugs. In fact, they are 400 times more likely to use hard drugs than those who do not have a family history of alcohol addiction.
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.
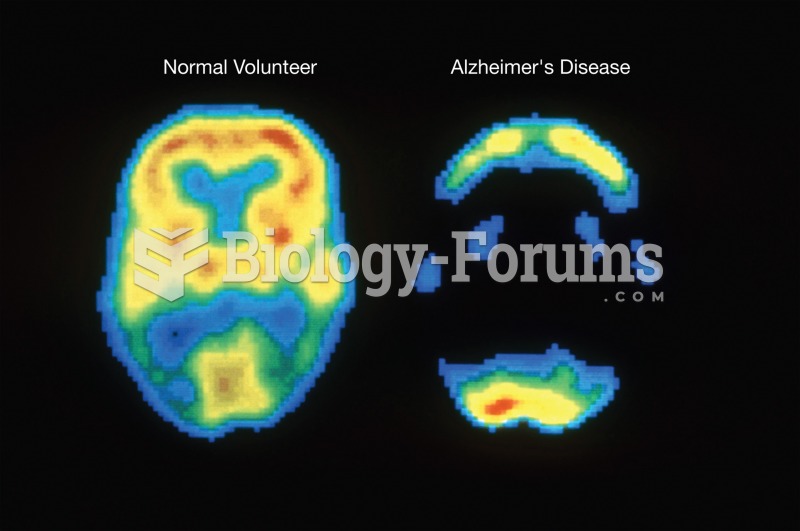 Positron emission tomography (PET) image showing the difference in the metabolic activity of the bra
Positron emission tomography (PET) image showing the difference in the metabolic activity of the bra
 Edward Bellamy, author of the utopian novel Looking Backward (1888) Bellamy’s socialism worried many
Edward Bellamy, author of the utopian novel Looking Backward (1888) Bellamy’s socialism worried many