This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Did you know?
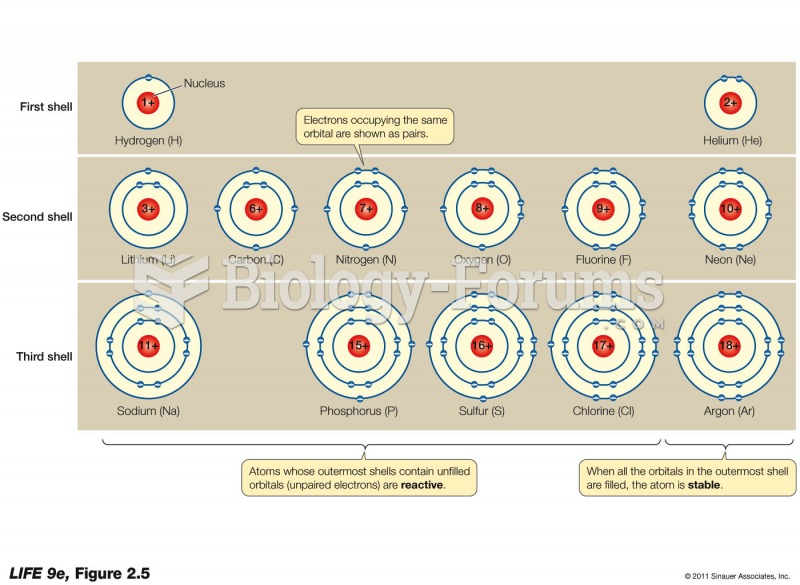
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.